Lab Med Online.
2022 Oct;12(4):285-291. 10.47429/lmo.2022.12.4.285.
Comparison of Trough-Based and Area Under the Curve-Based Therapeutic Drug Monitoring of Vancomycin: An In Silico Study
- Affiliations
-
- 1Department of Laboratory Medicine and Genetics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2538616
- DOI: http://doi.org/10.47429/lmo.2022.12.4.285
Abstract
- Background
Recent American guideline published in 2020 recommend using 24-h area under the curve (AUC)/minimum inhibitory concentration instead of vancomycin serum concentration (Ctrough) for vancomycin therapeutic drug monitoring (TDM). However, Ctrough-based TDM is widely used in clinical practice. Thus, this retrospective study aimed to compare Ctrough-based and AUC-based TDM.
Methods
We evaluated patients’ TDM data with at least one vancomycin trough measurement. Patients younger than 18 years, admitted to an intensive care unit, or on renal replacement therapy were excluded. The variables of Ctrough-based and AUC-based TDM were simulated using MwPharm++ (Mediware, Czech Republic) with vancomycin two-compartment model. The therapeutic range was 400-600 mg*h/L and 15-20 mg/L for AUC and Ctrough, respectively. We evaluated the correlation between Ctrough and AUC, the attainment rate of AUC target range, and changes in vancomycin dose and Ctrough when AUC-based TDM is applied.
Results
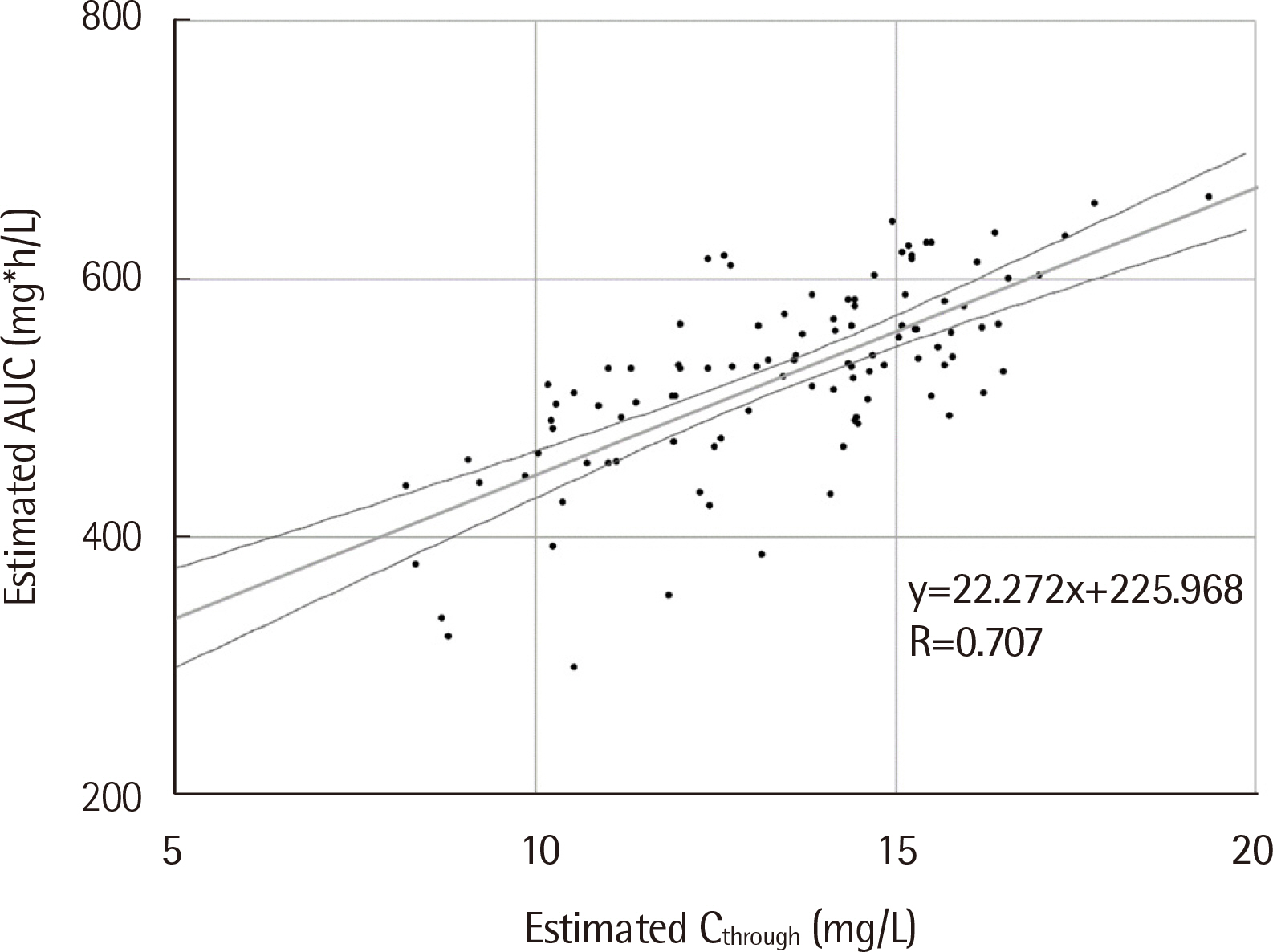
One hundred and four patients were enrolled. Ctrough and AUC correlated moderately (R=0.707, P<0.001). Among 31 patients with Ctrough of 15-20 mg/L, the AUC of only 18 patients was within the target range (18/31, 58.1%). In addition, most patients with Ctrough of 10-15 mg/L had the AUC within the target range (57/66, 86.4%). The respective vancomycin dose and Ctrough were expected to be significantly lower in AUC-based TDM simulation than those in Ctrough-based TDM simulation
Conclusions
Ctrough of 15-20 mg/L for vancomycin monitoring is not appropriate for attaining AUC target range. Targeting either AUC or lower Ctrough is recommended for vancomycin TDM.
Keyword
Figure
Reference
-
1. Blaskovich MAT, Hansford KA, Butler MS, Jia Z, Mark AE, Cooper MA. 2018; Developments in glycopeptide antibiotics. ACS Infect Dis. 4:715–35. DOI: 10.1021/acsinfecdis.7b00258. PMID: 29363950. PMCID: PMC5952257.
Article2. Rybak MJ. 2006; The pharmacokinetic and pharmacodynamic properties of vancomycin. Clin Infect Dis. 42(S1):S35–9. DOI: 10.1086/491712. PMID: 16323118.
Article3. Rybak MJ, Lomaestro BM, Rotschafer JC, Moellering RC Jr, Craig WA, Billeter M, et al. 2009; Therapeutic monitoring of vancomycin in adults summary of consensus recommendations from the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Pharmacotherapy. 29:1275–9. DOI: 10.1592/phco.29.11.1275. PMID: 19873687.4. Rybak MJ, Le J, Lodise TP, Levine DP, Bradley JS, Liu C, et al. 2020; Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: A revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 77:835–64. DOI: 10.1093/ajhp/zxaa036. PMID: 32191793.5. He N, Su S, Ye Z, Du G, He B, Li D, et al. 2020; Evidence-based guideline for therapeutic drug monitoring of vancomycin: 2020 update by the division of therapeutic drug monitoring, Chinese Pharmacological Society. Clin Infect Dis. 71(S4):S363–71.
Article6. Bradley N, Lee Y, Sadeia M. 2021; Assessment of the implementation of AUC dosing and monitoring practices with vancomycin at hospitals across the United States. J Pharm Pract. 8971900211012395. DOI: 10.1177/08971900211012395. PMID: 33902351.
Article7. Gregory ER, Burgess DR, Cotner SE, VanHoose JD, Flannery AH, Gardner B, et al. 2021; Pharmacist survey: Pharmacist perception of vancomycin area under the curve therapeutic drug monitoring. J Pharm Pract. 34:272–8. DOI: 10.1177/0897190019867494. PMID: 31422738.
Article8. Kufel WD, Seabury RW, Mogle BT, Beccari MV, Probst LA, Steele JM. 2019; Readiness to implement vancomycin monitoring based on area under the concentration-time curve: A cross-sectional survey of a national health consortium. Am J Health Syst Pharm. 76:889–94. DOI: 10.1093/ajhp/zxz070. PMID: 31063582.
Article9. Flannery AH, Hammond DA, Oyler DR, Li C, Wong A, Smith AP, et al. 2020; Vancomycin dosing practices among critical care pharmacists: A survey of Society of Critical Care Medicine Pharmacists. Infect Dis (Auckl). 13:1178633720952078. DOI: 10.1177/1178633720952078. PMID: 33029073. PMCID: PMC7522823.
Article10. Neef C, Touw DJ, Harteveld AR, Eerland JJ, Uges DR. 2006; Pitfalls in TDM of antibiotic drugs: Analytical and modelling issues. Ther Drug Monit. 28:686–9. DOI: 10.1097/01.ftd.0000243966.97964.11. PMID: 17038886.
Article11. Neely MN, Youn G, Jones B, Jelliffe RW, Drusano GL, Rodvold KA, et al. 2014; Are vancomycin trough concentrations adequate for optimal dosing? Antimicrob Agents Chemother. 58:309–16. DOI: 10.1128/AAC.01653-13. PMID: 24165176. PMCID: PMC3910745.
Article12. Hale CM, Seabury RW, Steele JM, Darko W, Miller CD. 2017; Are vancomycin trough concentrations of 15 to 20 mg/L associated with increased attainment of an AUC/MIC ≥ 400 in patients with presumed MRSA infection? J Pharm Pract. 30:329–35. DOI: 10.1177/0897190016642692. PMID: 27074786.
Article13. Clark L, Skrupky LP, Servais R, Brummitt CF, Dilworth TJ. 2019; Examining the relationship between vancomycin area under the concentration time curve and serum trough levels in adults with presumed or documented staphylococcal infections. Ther Drug Monit. 41:483–8. DOI: 10.1097/FTD.0000000000000622. PMID: 30817704.
Article14. Pai MP, Neely M, Rodvold KA, Lodise TP. 2014; Innovative approaches to optimizing the delivery of vancomycin in individual patients. Adv Drug Deliv Rev. 77:50–7. DOI: 10.1016/j.addr.2014.05.016. PMID: 24910345.
Article15. Marko R, Hajjar J, Nzeribe V, Pittman M, Deslandes V, Sant N, et al. 2021; Therapeutic drug monitoring of vancomycin in adult patients with methicillin-resistant Staphylococcus aureus bacteremia or pneumonia. Can J Hosp Pharm. 74:334–43. DOI: 10.4212/cjhp.v74i4.3195. PMID: 34602621. PMCID: PMC8463016.16. AbuSara AK, Abdelrahman DH, Habash KI, Al-Shaer MH, Le J, Nazer LH. 2022; Vancomycin therapeutic monitoring by measured trough concentration versus Bayesian-derived area under the curve in critically ill patients with cancer. Pharmacol Res Perspect. 10:e00912. DOI: 10.1002/prp2.912. PMID: 34990089. PMCID: PMC8929348.
Article17. Patel N, Pai MP, Rodvold KA, Lomaestro B, Drusano GL, Lodise TP. 2011; Vancomycin: We can't get there from here. Clin Infect Dis. 52:969–74. DOI: 10.1093/cid/cir078. PMID: 21460308.
Article18. Al-Sulaiti FK, Nader AM, Saad MO, Shaukat A, Parakadavathu R, Elzu-bair A, et al. 2019; Clinical and pharmacokinetic outcomes of peak-trough-based versus trough-based vancomycin therapeutic drug monitoring approaches: A pragmatic randomized controlled trial. Eur J Drug Metab Pharmacokinet. 44:639–52. DOI: 10.1007/s13318-019-00551-1. PMID: 30919233. PMCID: PMC6746691.
Article19. Suzuki A, Hamada Y, Ikeda H, Tanaka H, Yanagihara M, Namiki M, et al. 2021; Comparison of trough concentration and area under the curve of vancomycin associated with the incidence of nephrotoxicity and predictors of a high trough level. J Infect Chemother. 27:455–60. DOI: 10.1016/j.jiac.2020.10.014. PMID: 33144145.
Article20. Mogle BT, Steele JM, Seabury RW, Dang UJ, Kufel WD. 2018; Implementation of a two-point pharmacokinetic AUC-based vancomycin therapeutic drug monitoring approach in patients with methicillin-resistant Staphylococcus aureus bacteraemia. Int J Antimicrob Agents. 52:805–10. DOI: 10.1016/j.ijantimicag.2018.08.024. PMID: 30176357.21. Liu J, Tong SYC, Davis JS, Rhodes NJ, Scheetz MH. 2020; Vancomycin exposure and acute kidney injury outcome: A snapshot from the CAMERA2 study. Open Forum Infect Dis. 7:ofaa538. DOI: 10.1093/ofid/ofaa538. PMID: 33335938. PMCID: PMC7731530.
Article22. Lines J, Burchette J, Kullab SM, Lewis P. 2021; Evaluation of a trough-only extrapolated area under the curve vancomycin dosing method on clinical outcomes. Int J Clin Pharm. 43:263–9. DOI: 10.1007/s11096-020-01157-3. PMID: 32964405.
Article23. Rees MR, Carr DR, Trienski T, Buchanan C, White K, Bremmer DN. 2022; Outpatient vancomycin therapy: Acute kidney injury in individualized AUC-based goal trough ranges versus traditional trough dosing. J Am Pharm Assoc (2003). 62:706–10. DOI: 10.1016/j.japh.2021.11.031. PMID: 34920955.
Article24. Oda K, Jono H, Nosaka K, Saito H. 2020; Reduced nephrotoxicity with vancomycin therapeutic drug monitoring guided by area under the concentration-time curve against a trough 15-20 μg/mL concentration. Int J Antimicrob Agents. 56:106109. DOI: 10.1016/j.ijantimicag.2020.106109. PMID: 32721597.
Article25. Katip W, Okonogi S, Oberdorfer P. 2022; The thirty-day mortality rate and nephrotoxicity associated with trough serum vancomycin concentrations during treatment of enterococcal infections: A propensity score matching analysis. Front Pharmacol. 12:773994. DOI: 10.3389/fphar.2021.773994. PMID: 35153743. PMCID: PMC8831381.
Article26. Ueda T, Takesue Y, Nakajima K, Ichiki K, Ishikawa K, Yamada K, et al. 2022; Validation of vancomycin area under the concentration-time curve estimation by the Bayesian approach using one-point samples for predicting clinical outcomes in patients with methicillin-resistant Staphylococcus aureus infections. Antibiotics (Basel). 11:96. DOI: 10.3390/antibiotics11010096. PMID: 35052972. PMCID: PMC8772855.
Article27. Nix DE, Davis LE, Matthias KR. 2022; The relationship of vancomycin 24-hour AUC and trough concentration. Am J Health Syst Pharm. 79:534–9. DOI: 10.1093/ajhp/zxab457. PMID: 34849533.
Article28. Jager NGL, Chai MG, van Hest RM, Lipman J, Roberts JA, Cotta MO. Precision dosing software to optimise antimicrobial dosing: a systematic search and follow-up survey of available programs. Clin Microbiol Infect. 2022; doi: 10.1016/j.cmi.2022.03.041 (in press). DOI: 10.1016/j.cmi.2022.03.041. PMID: 35429656.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of Trough-Based and Area Under the Curve-Based Therapeutic Drug Monitoring of Vancomycin: An In Silico Study
- Underestimation of the Calculated Area Under the Concentration-Time Curve Based on Serum Creatinine for Vancomycin Dosing
- Evaluation of Vancomycin Area Under the Concentration–Time Curve Predictive Performance Using Bayesian Modeling Software With and Without Peak Concentration: An Academic Hospital Experience for Adult Patients Without Renal Impairment
- Assessment of Therapeutic Drug Monitoring of Vancomycin in Elderly Patients According to New Guidelines
- Appropriate Use of Glycopeptide Antibiotics and Therapeutic Drug Monitoring for Invasive Infections