Ann Lab Med.
2023 Nov;43(6):554-564. 10.3343/alm.2023.43.6.554.
Evaluation of Vancomycin Area Under the Concentration–Time Curve Predictive Performance Using Bayesian Modeling Software With and Without Peak Concentration: An Academic Hospital Experience for Adult Patients Without Renal Impairment
- Affiliations
-
- 1Department of Laboratory Medicine, University of Ulsan College of Medicine, Ulsan University Hospital, Ulsan, Korea
- 2Department of Laboratory Medicine, Ewha Womans University College of Medicine, Seoul, Korea
- KMID: 2552026
- DOI: http://doi.org/10.3343/alm.2023.43.6.554
Abstract
- Background
The revised U.S. consensus guidelines on vancomycin therapeutic drug monitoring (TDM) recommend obtaining trough and peak samples to estimate the area under the concentration–time curve (AUC) using the Bayesian approach; however, the benefit of such two-point measurements has not been demonstrated in a clinical setting. We evaluated Bayesian predictive performance with and without peak concentration data using clinical TDM data.
Methods
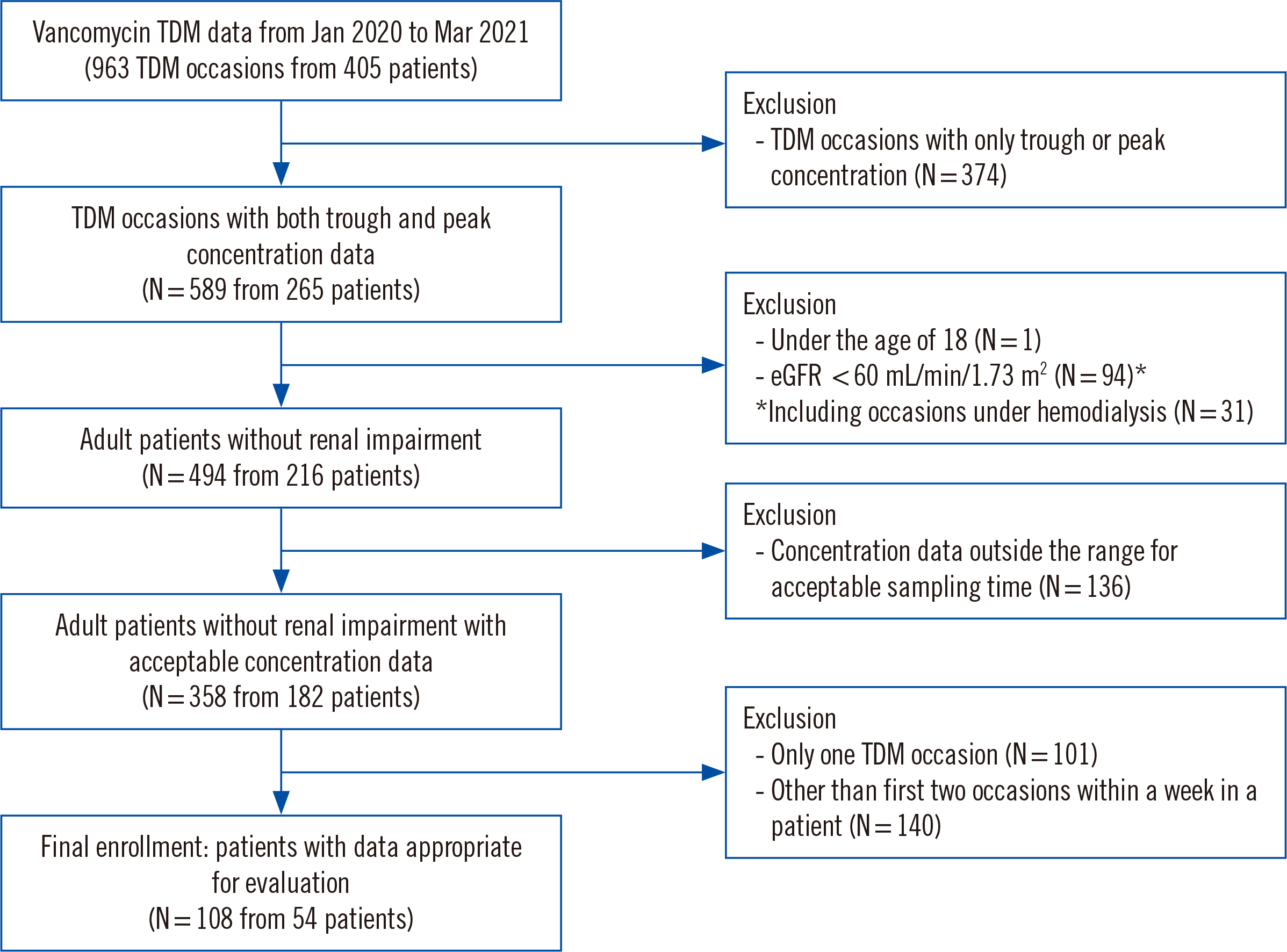
We retrospectively analyzed 54 adult patients without renal impairment who had two serial peak and trough concentration measurements in a ≤1-week interval. The concentration and AUC values were estimated and predicted using Bayesian software (MwPharm++; Mediware, Prague, Czech Republic). The median prediction error (MDPE) for bias and median absolute prediction error (MDAPE) for imprecision were calculated based on the estimated AUC and measured trough concentration.
Results
AUC predictions using the trough concentration had an MDPE of –1.6% and an MDAPE of 12.4%, whereas those using both peak and trough concentrations had an MDPE of –6.2% and an MDAPE of 16.9%. Trough concentration predictions using the trough concentration had an MDPE of –8.7% and an MDAPE of 18.0%, whereas those using peak and trough concentrations had an MDPE of –13.2% and an MDAPE of 21.0%.
Conclusions
The usefulness of the peak concentration for predicting the AUC on the next occasion by Bayesian modeling was not demonstrated; therefore, the practical value of peak sampling for AUC-guided dosing can be questioned. As this study was conducted in a specific setting and generalization is limited, results should be interpreted cautiously.
Figure
Cited by 1 articles
-
Survey on the Current Status of Therapeutic Drug Monitoring and Vancomycin Pharmacokinetic Consultation Service in Clinical Laboratories in Korea
Mikyoung Park, Hyun-Ki Kim, Jong Do Seo, Tae-Dong Jeong, Misuk Ji
Lab Med Online. 2025;15(1):58-69. doi: 10.47429/lmo.2025.15.1.58.
Reference
-
1. Turner NA, Sharma-Kuinkel BK, Maskarinec SA, Eichenberger EM, Shah PP, Carugati M, et al. 2019; Methicillin-resistant Staphylococcus aureus: an overview of basic and clinical research. Nat Rev Microbiol. 17:203–18. DOI: 10.1038/s41579-018-0147-4. PMID: 30737488. PMCID: PMC6939889.
Article2. Rybak M, Lomaestro B, Rotschafer JC, Moellering R Jr, Craig W, Billeter M, et al. 2009; Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 66:82–98. DOI: 10.2146/ajhp080434. PMID: 19106348.
Article3. Revilla N, Martín-Suárez A, Pérez MP, González Gonzalez FM, Fernández de Gatta MM. 2010; Vancomycin dosing assessment in intensive care unit patients based on a population pharmacokinetic/pharmacodynamic simulation. Br J Clin Pharmacol. 70:201–12. DOI: 10.1111/j.1365-2125.2010.03679.x. PMID: 20653673. PMCID: PMC2911550.
Article4. Rybak MJ, Le J, Lodise TP, Levine DP, Bradley JS, Liu C, et al. 2020; Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 77:835–64. DOI: 10.1093/ajhp/zxaa036. PMID: 32191793.5. Reuter SE, Stocker SL, Alffenaar JC, Baldelli S, Cattaneo D, Jones G, et al. 2022; Optimal practice for vancomycin therapeutic drug monitoring: position statement from the Anti-Infectives Committee of the International Association of Therapeutic Drug Monitoring and Clinical Toxicology. Ther Drug Monit. 44:121–32. DOI: 10.1097/FTD.0000000000000944. PMID: 34882107.
Article6. van der Meer AF, Touw DJ, Marcus MA, Neef C, Proost JH. 2012; Influence of erroneous patient records on population pharmacokinetic modeling and individual bayesian estimation. Ther Drug Monit. 34:526–34. DOI: 10.1097/FTD.0b013e3182616937. PMID: 22846895.
Article7. Matsumoto K, Oda K, Shoji K, Hanai Y, Takahashi Y, Fujii S, et al. 2022; Clinical practice guidelines for therapeutic drug monitoring of vancomycin in the framework of model-informed precision dosing: a consensus review by the Japanese society of chemotherapy and the Japanese society of therapeutic drug monitoring. Pharmaceutics. 14:489. DOI: 10.3390/pharmaceutics14030489. PMID: 35335866. PMCID: PMC8955715.
Article8. Neely MN, Youn G, Jones B, Jelliffe RW, Drusano GL, Rodvold KA, et al. 2014; Are vancomycin trough concentrations adequate for optimal dosing? Antimicrob Agents Chemother. 58:309–16. DOI: 10.1128/AAC.01653-13. PMID: 24165176. PMCID: PMC3910745.
Article9. Turner RB, Kojiro K, Shephard EA, Won R, Chang E, Chan D, et al. 2018; Review and validation of bayesian dose-optimizing software and equations for calculation of the vancomycin area under the curve in critically ill patients. Pharmacotherapy. 38:1174–83. DOI: 10.1002/phar.2191. PMID: 30362592.
Article10. Oda K, Hashiguchi Y, Kimura T, Tsuji Y, Shoji K, Takahashi Y, et al. 2021; Performance of area under the concentration-time curve estimations of vancomycin with limited sampling by a newly developed web application. Pharm Res. 38:637–46. DOI: 10.1007/s11095-021-03030-y. PMID: 33782837.
Article11. Alsowaida YS, Kubiak DW, Dionne B, Kovacevic MP, Pearson JC. 2022; Vancomycin area under the concentration-time curve estimation using bayesian modeling versus first-order pharmacokinetic equations: a quasi-experimental study. Antibiotics (Basel). 11:1239. DOI: 10.3390/antibiotics11091239. PMID: 36140021. PMCID: PMC9495010.
Article12. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. 2009; A new equation to estimate glomerular filtration rate. Ann Intern Med. 150:604–12. DOI: 10.7326/0003-4819-150-9-200905050-00006. PMID: 19414839. PMCID: PMC2763564.
Article13. Pai MP, Neely M, Rodvold KA, Lodise TP. 2014; Innovative approaches to optimizing the delivery of vancomycin in individual patients. Adv Drug Deliv Rev. 77:50–7. DOI: 10.1016/j.addr.2014.05.016. PMID: 24910345.
Article14. Neef C, Touw DJ, Harteveld AR, Eerland JJ, Uges DR. 2006; Pitfalls in TDM of antibiotic drugs: analytical and modelling issues. Ther Drug Monit. 28:686–9. DOI: 10.1097/01.ftd.0000243966.97964.11. PMID: 17038886.
Article15. Cockcroft DW, Gault MH. 1976; Prediction of creatinine clearance from serum creatinine. Nephron. 16:31–41. DOI: 10.1159/000180580. PMID: 1244564.
Article16. Waikar SS, Bonventre JV. 2009; Creatinine kinetics and the definition of acute kidney injury. J Am Soc Nephrol. 20:672–9. DOI: 10.1681/ASN.2008070669. PMID: 19244578. PMCID: PMC2653692.
Article17. Jelliffe RW, Schumitzky A, Guilder M. 1992; Nonpharmacokinetic clinical factors affecting aminoglycoside therapeutic precision. Drug Invest. 4:20–9. DOI: 10.1007/BF03258374.
Article18. Alihodzic D, Broeker A, Baehr M, Kluge S, Langebrake C, Wicha SG. 2020; Impact of inaccurate documentation of sampling and infusion time in model-informed precision dosing. Front Pharmacol. 11:172. DOI: 10.3389/fphar.2020.00172. PMID: 32194411. PMCID: PMC7063976.
Article19. Olney KB, Wallace KL, Mynatt RP, Burgess DS, Grieves K, Willett A, et al. 2022; Comparison of Bayesian-derived and first-order analytic equations for calculation of vancomycin area under the curve. Pharmacotherapy. 42:284–91. DOI: 10.1002/phar.2670. PMID: 35134264. PMCID: PMC9750735.
Article20. Narayan SW, Thoma Y, Drennan PG, Yejin Kim H, Alffenaar JW, Van Hal S, et al. 2021; Predictive performance of bayesian vancomycin monitoring in the critically Ill. Crit Care Med. 49:e952–e60. DOI: 10.1097/CCM.0000000000005062. PMID: 33938713.
Article21. Cunio CB, Uster DW, Carland JE, Buscher H, Liu Z, Brett J, et al. 2020; Towards precision dosing of vancomycin in critically ill patients: an evaluation of the predictive performance of pharmacometric models in ICU patients. Clin Microbiol Infect. 27:S1198-743X(20)30388-8783e7-e14. DOI: 10.1016/j.cmi.2020.07.005. PMID: 32673799.
Article22. Broeker A, Nardecchia M, Klinker KP, Derendorf H, Day RO, Marriott DJ, et al. 2019; Towards precision dosing of vancomycin: a systematic evaluation of pharmacometric models for Bayesian forecasting. Clin Microbiol Infect. 25:1286.e1–e7. DOI: 10.1016/j.cmi.2019.02.029. PMID: 30872102.
Article23. Roydhouse SA, Carland JE, Debono DS, Baysari MT, Reuter SE, Staciwa AJ, et al. 2021; Accuracy of documented administration times for intravenous antimicrobial drugs and impact on dosing decisions. Br J Clin Pharmacol. 87:4273–82. DOI: 10.1111/bcp.14844. PMID: 33792079.
Article24. Melanson SEF, Mijailovic AS, Wright APM, Szumita PM, Bates DW, Tanasijevic MJ. 2013; An intervention to improve the timing of vancomycin levels. Am J Clin Pathol. 140:801–6. DOI: 10.1309/AJCPKQ6EAH7OYQLB. PMID: 24225746.
Article25. Neely MN, Kato L, Youn G, Kraler L, Bayard D, van Guilder M, et al. 2018; Prospective trial on the use of trough concentration versus area under the curve to determine therapeutic vancomycin dosing. Antimicrob Agents Chemother. 62:e02042–17. DOI: 10.1128/AAC.02042-17. PMID: 29203493. PMCID: PMC5786789.
Article26. Brocks DR, Hamdy DA. 2020; Bayesian estimation of pharmacokinetic parameters: an important component to include in the teaching of clinical pharmacokinetics and therapeutic drug monitoring. Res Pharm Sci. 15:503–14. DOI: 10.4103/1735-5362.301335. PMID: 33828594. PMCID: PMC8020855.
Article27. Kim HK, Park HD, Lee SG, Chae H, Song SH, Lee YW, et al. 2021; Immunosuppressive drug measurement by liquid chromatography coupled to tandem mass spectrometry: interlaboratory comparison in the Korean clinical laboratories. Ann Lab Med. 41:268–76. DOI: 10.3343/alm.2021.41.3.268. PMID: 33303711. PMCID: PMC7748092.
Article28. Choi R, Chun MR, Park J, Lee JW, Ju HY, Cho HW, et al. 2021; Quantification of thioguanine in DNA using liquid chromatography-tandem mass spectrometry for routine thiopurine drug monitoring in patients with pediatric acute lymphoblastic leukemia. Ann Lab Med. 41:145–54. DOI: 10.3343/alm.2021.41.2.145. PMID: 33063676. PMCID: PMC7591283.
Article29. Sonoda A, Iwashita Y, Takada Y, Hamazono R, Ishida K, Imamura H. 2022; Prediction accuracy of area under the concentration-time curve of vancomycin by Bayesian approach using creatinine-based equations of estimated kidney function in bedridden elderly Japanese patients. Biol Pharm Bull. 45:763–9. DOI: 10.1248/bpb.b22-00070. PMID: 35370223.
Article30. Choi R, Woo HI, Park HD, Lee SY. 2019; A nationwide utilization survey of therapeutic drug monitoring for five antibiotics in South Korea. Infect Drug Resist. 12:2163–73. DOI: 10.2147/IDR.S208783. PMID: 31410036. PMCID: PMC6646174.31. Lim HS, Chong YP, Noh YH, Jung JA, Kim YS. 2014; Exploration of optimal dosing regimens of vancomycin in patients infected with methicillin-resistant Staphylococcus aureus by modeling and simulation. J Clin Pharm Ther. 39:196–203. DOI: 10.1111/jcpt.12123. PMID: 24428720.
Article32. Kim DJ, Lee DH, Ahn S, Jung J, Kiem S, Kim SW, et al. 2019; A new population pharmacokinetic model for vancomycin in patients with variable renal function: therapeutic drug monitoring based on extended covariate model using CKD-EPI estimation. J Clin Pharm Ther. 44:750–9. DOI: 10.1111/jcpt.12995. PMID: 31228353.
Article33. Bae SH, Yim DS, Lee H, Park AR, Kwon JE, Sumiko H, et al. Application of pharmacometrics in pharmacotherapy: open-source software for vancomycin therapeutic drug management. Pharmaceutics. 2019; 11:DOI: 10.3390/pharmaceutics11050224. PMID: 31075931. PMCID: PMC6572512.
Article34. Lee BV, Fong G, Bolaris M, Neely M, Minejima E, Kang A, et al. 2021; Cost-benefit analysis comparing trough, two-level AUC and Bayesian AUC dosing for vancomycin. Clin Microbiol Infect. 27:1346.e1–e7. DOI: 10.1016/j.cmi.2020.11.008. PMID: 33221430.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Underestimation of the Calculated Area Under the Concentration-Time Curve Based on Serum Creatinine for Vancomycin Dosing
- Comparison of Trough-Based and Area Under the Curve-Based Therapeutic Drug Monitoring of Vancomycin: An In Silico Study
- Comparisons of predictive modeling techniques for breast cancer in Korean women
- Evaluation of the Effect of Initial dose of Vancomycin using Serum Cystatin C as a Marker in Elderly Patients
- Interinstitutional Comparison of Vancomycin Area Under the Concentration–Time Curve Estimation in Korea: Need for Standardized Operational Protocols for Therapeutic Drug Monitoring Consultation