Cancer Res Treat.
2023 Jan;55(1):270-278. 10.4143/crt.2021.1537.
The Efficacy of Alternate Systemic Intravenous Chemotherapy and Intra-arterial Chemotherapy Approach for Eye Globe Salvage in Retinoblastoma
- Affiliations
-
- 1Division of Pediatric Hematology and Oncology, Department of Pediatrics, Yonsei University Health System, Yonsei University College of Medicine, Seoul, Korea
- 2Department of Pediatric Hemato-Oncology, Yonsei Cancer Center, Yonsei University Health System, Seoul, Korea
- 3Department of Ophthalmology, The Institute of Vision Research, Yonsei University College of Medicine, Seoul, Korea
- 4Department of Radiology, Yonsei University College of Medicine, Seoul, Korea
- KMID: 2538010
- DOI: http://doi.org/10.4143/crt.2021.1537
Abstract
- Purpose
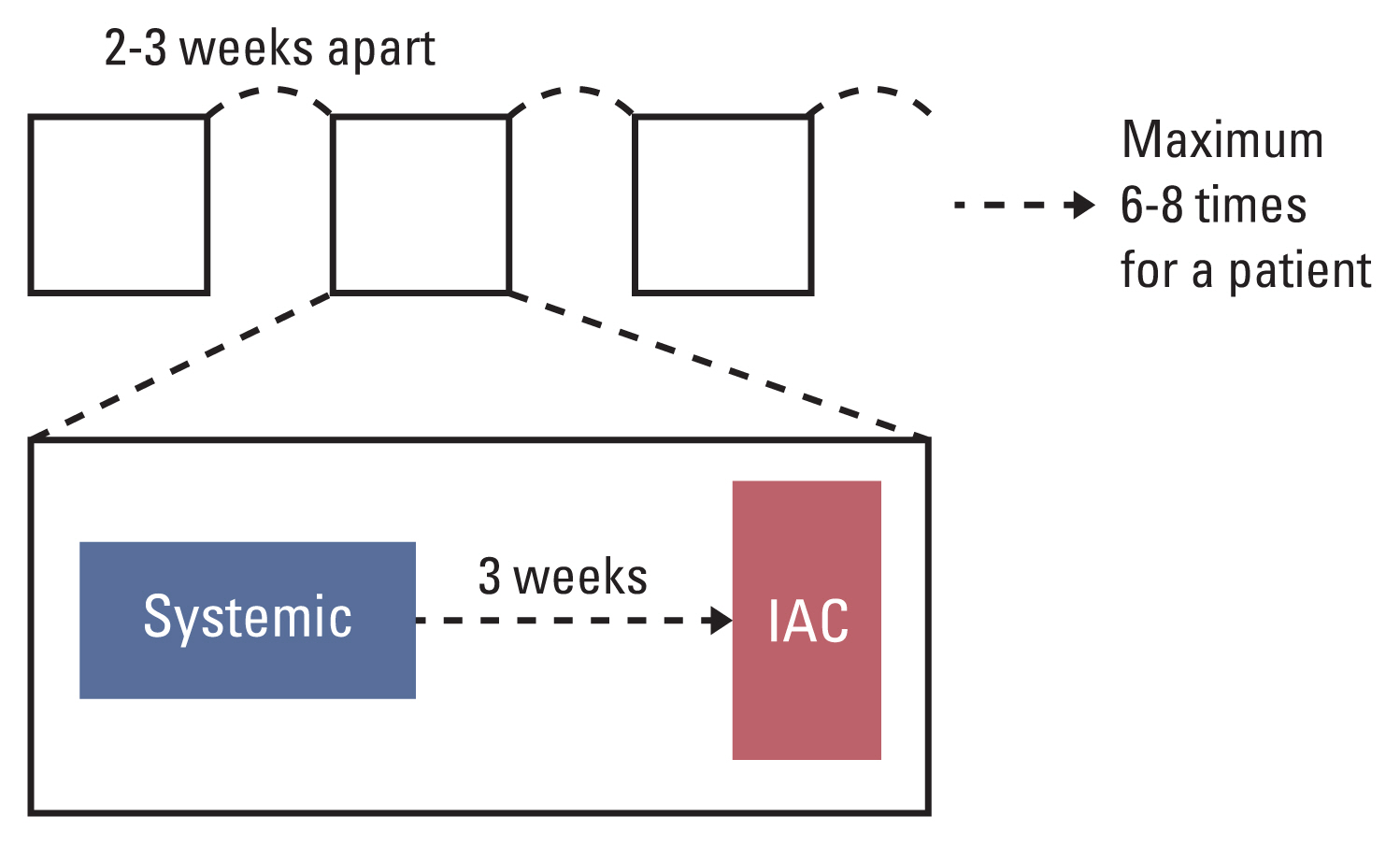
The advances in the treatment of retinoblastoma have enabled salvaging the globe in advanced stages with intra-arterial chemotherapy (IAC). We developed a strategy of alternate application of systemic intravenous chemotherapy (IVC) and IAC (referred to as alternate systemic IVC and IAC; ASIAC) to reduce central nervous metastases during IAC and examined its efficacy and safety in eye globe salvage in this study.
Materials and Methods
Between January 2010 and February 2021, 43 eyes of 40 patients received ASIAC treatment for retinoblastoma at the Yonsei Cancer Center, Yonsei University Health System. Their medical records were reviewed retrospectively to evaluate the eye salvage rate (ESR), defined from diagnosis to enucleation. High-risk retinoblastoma was defined as group D or E by the International Classification of Retinoblastoma.
Results
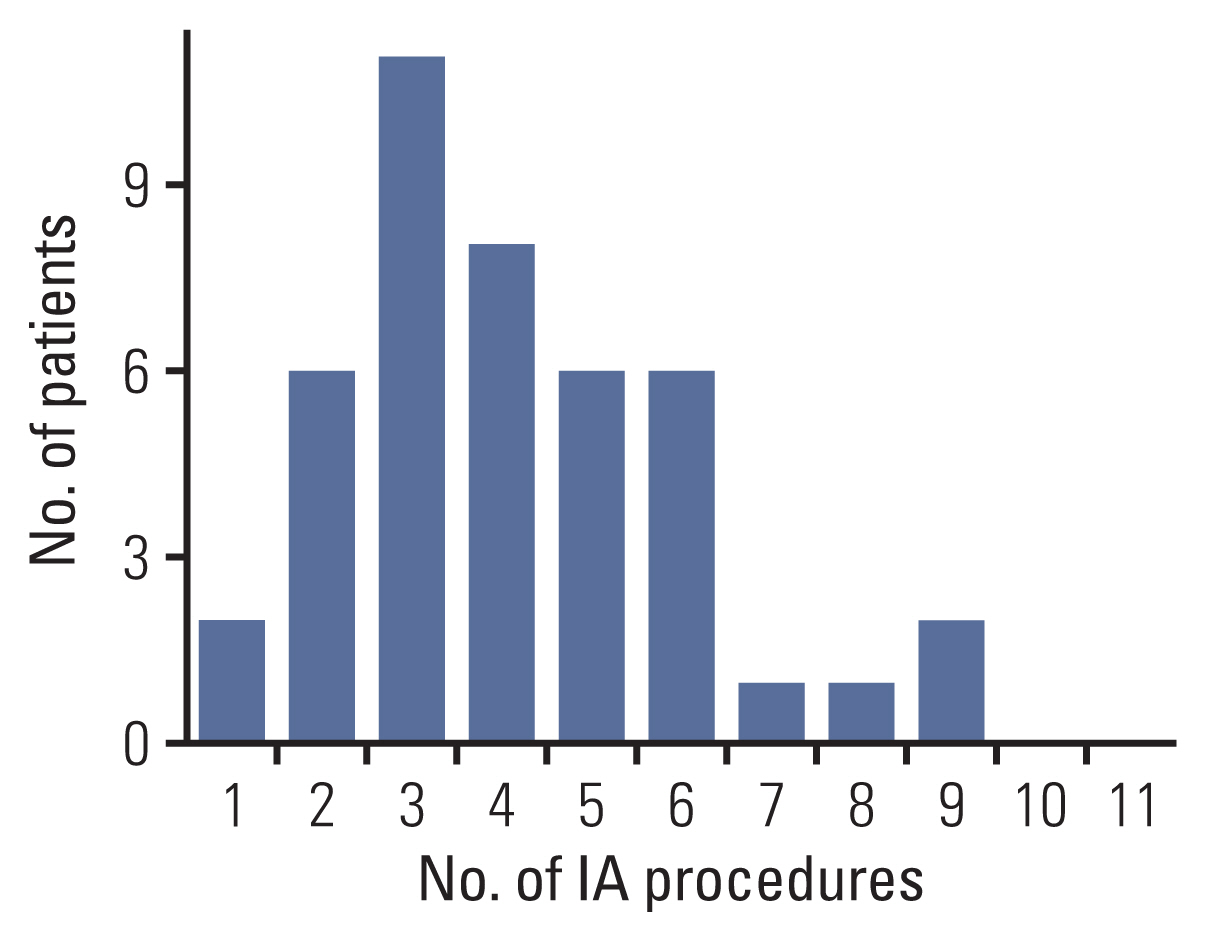
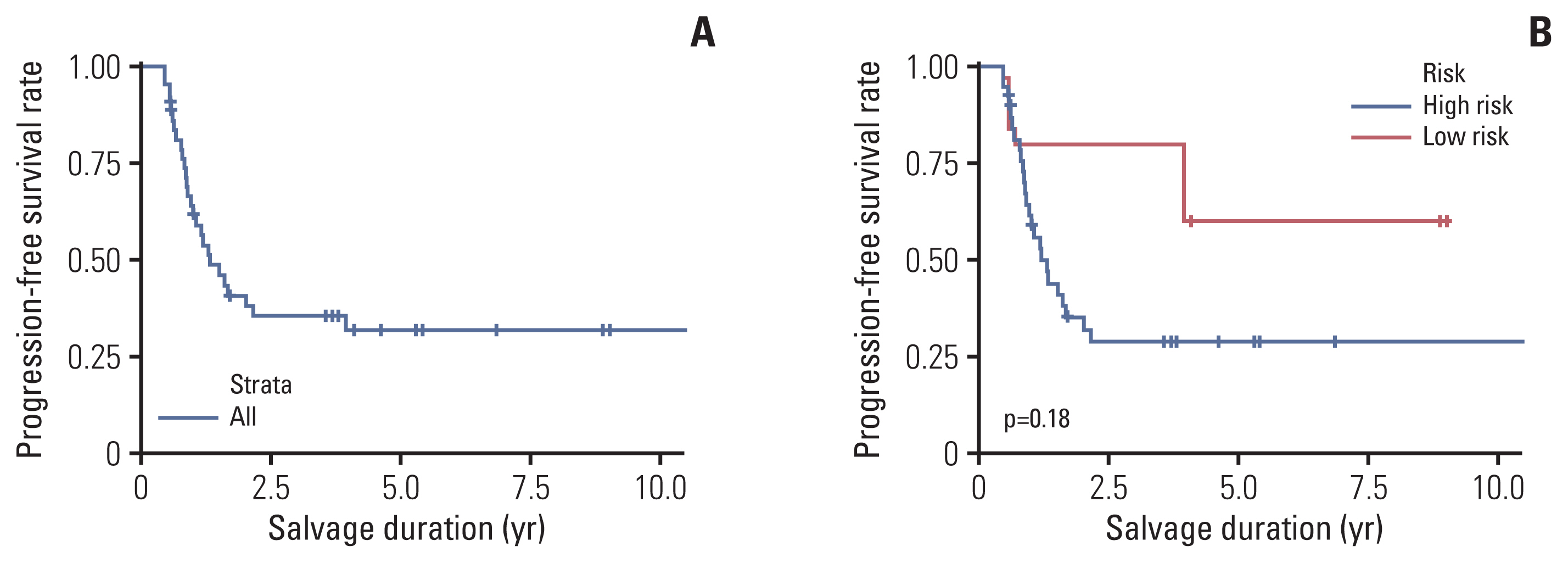
The study enrolled 38 and five cases of high-risk and low-risk retinoblastoma, respectively. In total, 178 IAC and 410 IVC courses were administered, with a median of 4 (interquartile range [IQR], 3.0 to 5.0) IAC and 9 (IQR, 6.0 to 11) IVC courses per eye, respectively. The 5-year ESR was 60.4%±8.7% for the whole cohort, 100% for low-risk retinoblastoma, and 53.6%±9.8% for high-risk retinoblastoma. Among those diagnosed since 2015, the 5-year ESR for high-risk retinoblastoma was 63.5%±14.0%. Fifteen eyes underwent enucleation; no viable tumor was found in three enucleated eyes. There were no deaths in this cohort.
Conclusion
Primary IAC-IVC (i.e., ASIAC) for patients with retinoblastoma was tolerable and effective in salvaging the eye and maintaining survival.
Figure
Reference
-
References
1. Dimaras H, Kimani K, Dimba EA, Gronsdahl P, White A, Chan HS, et al. Retinoblastoma. Lancet. 2012; 379:1436–46.2. Abramson DH, Shields CL, Munier FL, Chantada GL. Treatment of retinoblastoma in 2015: agreement and disagreement. JAMA Ophthalmol. 2015; 133:1341–7.3. Kim JW, Abramson DH, Dunkel IJ. Current management strategies for intraocular retinoblastoma. Drugs. 2007; 67:2173–85.4. Kim H, Lee JW, Kang HJ, Park HJ, Kim YY, Shin HY, et al. Clinical results of chemotherapy based treatment in retinoblastoma patients: a single center experience. Cancer Res Treat. 2008; 40:164–71.5. Gobin YP, Dunkel IJ, Marr BP, Brodie SE, Abramson DH. Intra-arterial chemotherapy for the management of retinoblastoma: four-year experience. Arch Ophthalmol. 2011; 129:732–7.6. Shields CL, Manjandavida FP, Lally SE, Pieretti G, Arepa-lli SA, Caywood EH, et al. Intra-arterial chemotherapy for retinoblastoma in 70 eyes: outcomes based on the international classification of retinoblastoma. Ophthalmology. 2014; 121:1453–60.7. Suzuki S, Kaneko A. Management of intraocular retinoblastoma and ocular prognosis. Int J Clin Oncol. 2004; 9:1–6.8. Ravindran K, Dalvin LA, Pulido JS, Brinjikji W. Intra-arterial chemotherapy for retinoblastoma: an updated systematic review and meta-analysis. J Neurointerv Surg. 2019; 11:1266–72.9. Yousef YA, Soliman SE, Astudillo PP, Durairaj P, Dimaras H, Chan HS, et al. Intra-arterial chemotherapy for retinoblastoma: a systematic review. JAMA Ophthalmol. 2016; 134:584–91.10. Khelfaoui F, Validire P, Auperin A, Quintana E, Michon J, Pacquement H, et al. Histopathologic risk factors in retinoblastoma: a retrospective study of 172 patients treated in a single institution. Cancer. 1996; 77:1206–13.11. Singh L, Kashyap S. Update on pathology of retinoblastoma. Int J Ophthalmol. 2018; 11:2011–6.12. Sastre X, Chantada GL, Doz F, Wilson MW, de Davila MT, Rodriguez-Galindo C, et al. Proceedings of the consensus meetings from the International Retinoblastoma Staging Working Group on the pathology guidelines for the examination of enucleated eyes and evaluation of prognostic risk factors in retinoblastoma. Arch Pathol Lab Med. 2009; 133:1199–202.13. Kaliki S, Shields CL, Shah SU, Eagle RC Jr, Shields JA, Leahey A. Postenucleation adjuvant chemotherapy with vincristine, etoposide, and carboplatin for the treatment of high-risk retinoblastoma. Arch Ophthalmol. 2011; 129:1422–7.14. Chevez-Barrios P, Eagle RC Jr, Krailo M, Piao J, Albert DM, Gao Y, et al. Study of unilateral retinoblastoma with and without histopathologic high-risk features and the role of adjuvant chemotherapy: a Children’s Oncology Group study. J Clin Oncol. 2019; 37:2883–91.15. Pekacka A. The role of intraarterial chemotherapy in the management of retinoblastoma. J Ophthalmol. 2020; 2020:3638410.16. Jabbour P, Chalouhi N, Tjoumakaris S, Gonzalez LF, Dumont AS, Chitale R, et al. Pearls and pitfalls of intraarterial chemotherapy for retinoblastoma. J Neurosurg Pediatr. 2012; 10:175–81.17. Hahn SM, Kim HS, Kim DJ, Lee SC, Lyu CJ, Han JW. Favorable outcome of alternate systemic and intra-arterial chemotherapy for retinoblastoma. Pediatr Hematol Oncol. 2016; 33:74–82.18. Choi S, Han JW, Kim H, Kim BS, Kim DJ, Lee SC, et al. Combined chemotherapy and intra-arterial chemotherapy of retinoblastoma. Korean J Pediatr. 2013; 56:254–9.19. Lee DH, Han JW, Hahn SM, Kim BM, Lyu CJ, Lee SC, et al. Changes in treatment patterns and globe salvage rate of advanced retinoblastoma in Korea: efficacy of intra-arterial chemotherapy. J Clin Med. 2021; 10:5421.20. Berry JL, Munier FL, Gallie BL, Polski A, Shah S, Shields CL, et al. Response criteria for intraocular retinoblastoma: RB-RECIST. Pediatr Blood Cancer. 2021; 68:e28964.21. Chen Q, Zhang B, Dong Y, Mo X, Zhang L, Xia J, et al. Evaluating primary intra-arterial chemotherapy versus intravenous plus intra-arterial chemotherapy for advanced intraocular retinoblastoma. Cancer Chemother Pharmacol. 2020; 85:723–30.22. Xu K, Liu J, Zhang C. Intra-arterial chemotherapy combined with VEC intravenous chemotherapy in the treatment of advanced retinoblastoma. J BUON. 2020; 25:1199–205.23. Marr BP, Brodie SE, Dunkel IJ, Gobin YP, Abramson DH. Three-drug intra-arterial chemotherapy using simultaneous carboplatin, topotecan and melphalan for intraocular retinoblastoma: preliminary results. Br J Ophthalmol. 2012; 96:1300–3.24. Shields CL, Alset AE, Say EA, Caywood E, Jabbour P, Shields JA. Retinoblastoma control with primary intra-arterial chemotherapy: outcomes before and during the intravitreal chemotherapy era. J Pediatr Ophthalmol Strabismus. 2016; 53:275–84.25. Grigorovski N, Lucena E, Mattosinho C, Parareda A, Ferman S, Catala J, et al. Use of intra-arterial chemotherapy for retinoblastoma: results of a survey. Int J Ophthalmol. 2014; 7:726–30.26. Scelfo C, Francis JH, Khetan V, Jenkins T, Marr B, Abramson DH, et al. An international survey of classification and treatment choices for group D retinoblastoma. Int J Ophthalmol. 2017; 10:961–7.27. Monroy JE, Orbach DB, VanderVeen D. Complications of intra-arterial chemotherapy for retinoblastoma. Semin Ophthalmol. 2014; 29:429–33.28. de Graaf P, Goricke S, Rodjan F, Galluzzi P, Maeder P, Castelijns JA, et al. Guidelines for imaging retinoblastoma: imaging principles and MRI standardization. Pediatr Radiol. 2012; 42:2–14.29. Shields CL, Palamar M, Sharma P, Ramasubramanian A, Leahey A, Meadows AT, et al. Retinoblastoma regression patterns following chemoreduction and adjuvant therapy in 557 tumors. Arch Ophthalmol. 2009; 127:282–90.30. Friedman DL, Krailo M, Villaluna D, Gombos D, Langholz B, Jubran R, et al. Systemic neoadjuvant chemotherapy for Group B intraocular retinoblastoma (ARET0331): a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2017; 64:e26394.31. Ghassemi F, Rahmanikhah E, Roohipoor R, Karkhaneh R, Faegh A. Regression patterns in treated retinoblastoma with chemotherapy plus focal adjuvant therapy. Pediatr Blood Cancer. 2013; 60:599–604.32. Francis JH, Levin AM, Zabor EC, Gobin YP, Abramson DH. Ten-year experience with ophthalmic artery chemosurgery: ocular and recurrence-free survival. PLoS One. 2018; 13:e0197081.33. Global Retinoblastoma Study Group, Fabian ID, Abdallah E, Abdullahi SU, Abdulqader RA, Adamou Boubacar S, et al. Global retinoblastoma presentation and analysis by national income level. JAMA Oncol. 2020; 6:685–95.34. Park SJ, Woo SJ, Park KH. Incidence of retinoblastoma and survival rate of retinoblastoma patients in Korea using the Korean National Cancer Registry database (1993–2010). Invest Ophthalmol Vis Sci. 2014; 55:2816–21.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Combined chemotherapy and intra-arterial chemotherapy of retinoblastoma
- Current Assessment and Management of Retinoblastoma
- Eye-Preserving Therapy in Retinoblastoma: Prolonged Primary Chemotherapy Alone or Combined with Local Therapy
- Diagnosis & Treatment of Retinoblastoma: Current Review
- Chemoreduction Followed by Local Therapy and Adjuvant Chemotherapy for Advanced Intraocular Retinoblastoma: A Pilot Study in a Single Center