Diabetes Metab J.
2022 Nov;46(6):879-889. 10.4093/dmj.2021.0265.
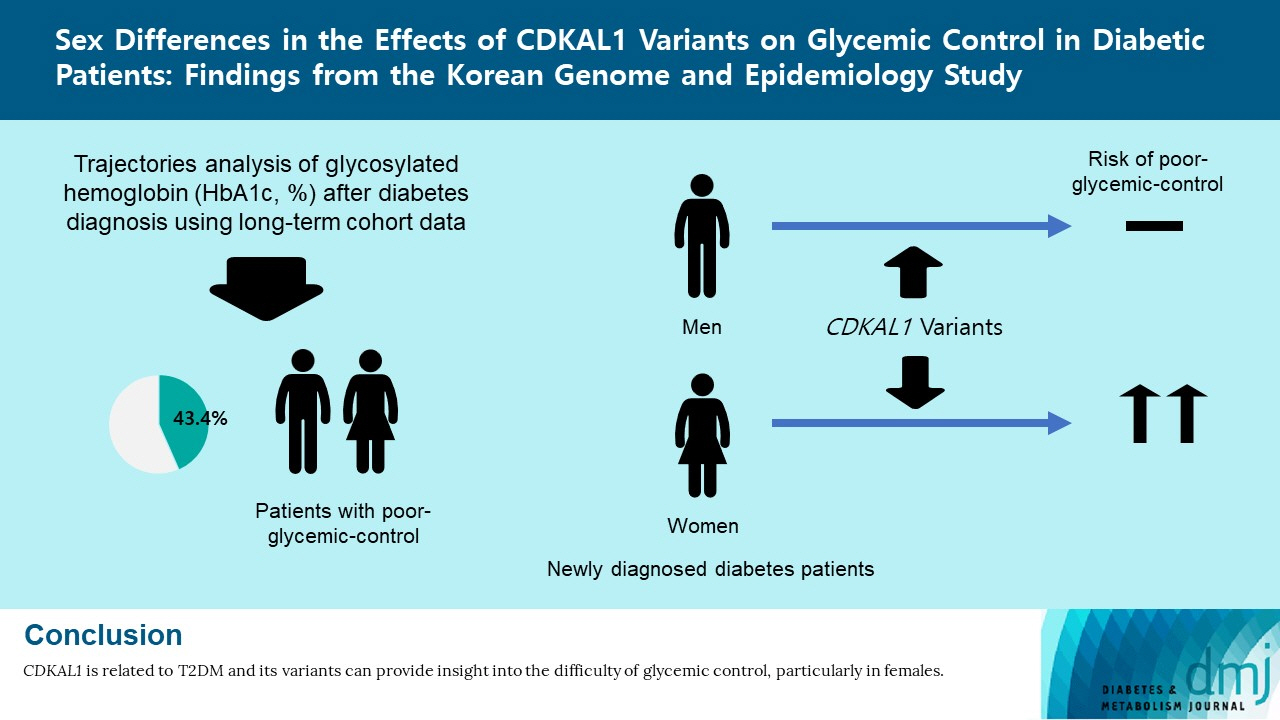
Sex Differences in the Effects of CDKAL1 Variants on Glycemic Control in Diabetic Patients: Findings from the Korean Genome and Epidemiology Study
- Affiliations
-
- 1Clinical Trial Center, Ewha Womans University Mokdong Hospital, Seoul, Korea
- 2Department of Preventive Medicine, Ewha Womans University College of Medicine, Seoul, Korea
- 3Graduate Program in System Health Science and Engineering, Ewha Womans University, Seoul, Korea
- 4Department of Internal Medicine, Ewha Womans University College of Medicine, Seoul, Korea
- KMID: 2536146
- DOI: http://doi.org/10.4093/dmj.2021.0265
Abstract
- Background
Using long-term data from the Korean Genome and Epidemiology Study, we defined poor glycemic control and investigated possible risk factors, including variants related to type 2 diabetes mellitus (T2DM). In addition, we evaluated interaction effects among risk factors for poor glycemic control.
Methods
Among 436 subjects with newly diagnosed diabetes, poor glycemic control was defined based on glycosylated hemoglobin trajectory patterns by group-based trajectory modeling. For the variants related to T2DM, genetic risk scores (GRSs) were calculated and divided into quartiles. Risk factors for poor glycemic control were assessed using a logistic regression model.
Results
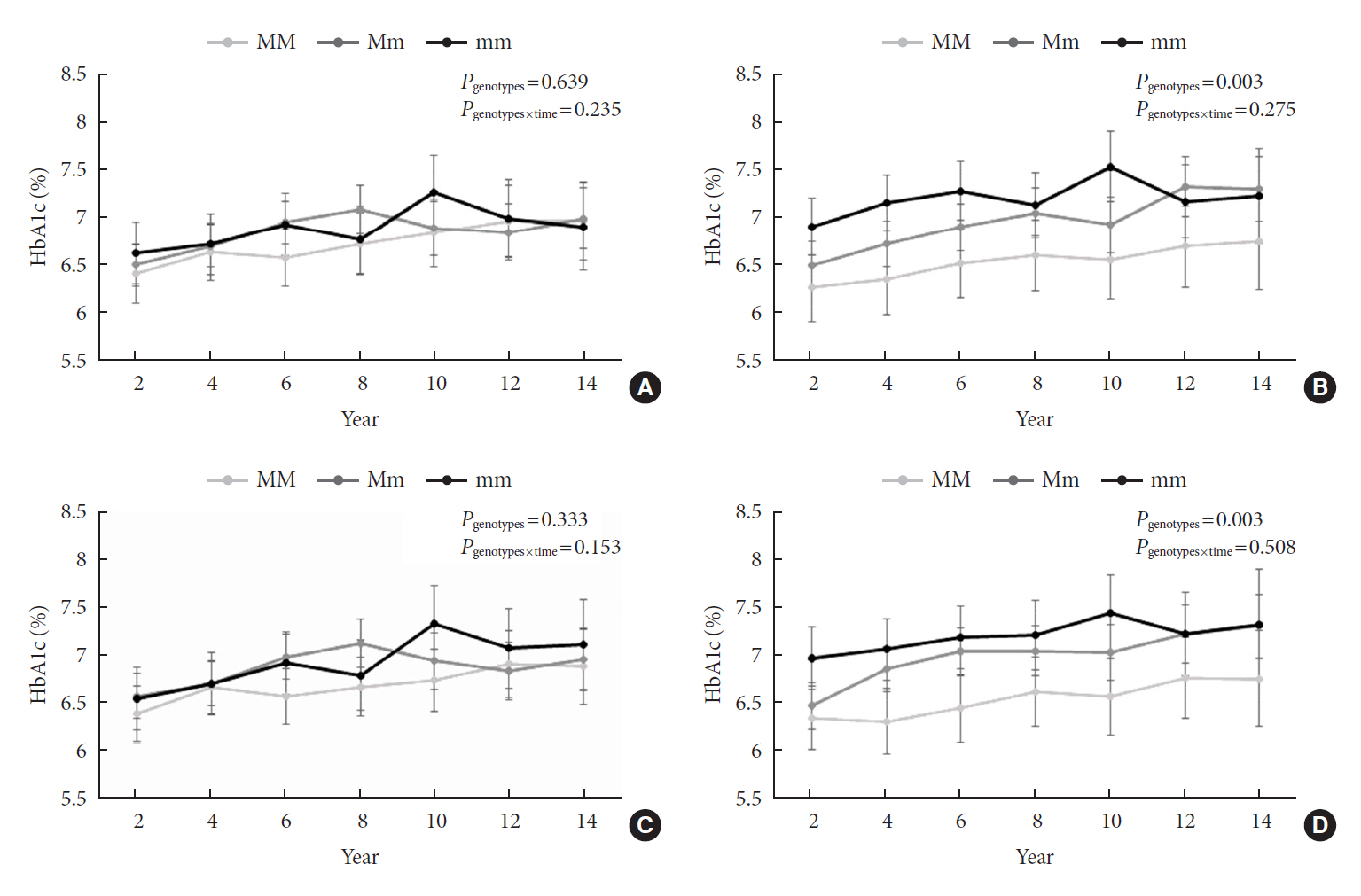
Of the subjects, 43% were in the poor-glycemic-control group. Body mass index (BMI) and triglyceride (TG) were associated with poor glycemic control. The risk for poor glycemic control increased by 11.0% per 1 kg/m2 increase in BMI and by 3.0% per 10 mg/dL increase in TG. The risk for GRS with poor glycemic control was sex-dependent (Pinteraction=0.07), and a relationship by GRS quartiles was found in females but not in males. Moreover, the interaction effect was found to be significant on both additive and multiplicative scales. The interaction effect was evident in the variants of cyclin-dependent kinase 5 regulatory subunit-associated protein 1-like (CDKAL1).
Conclusion
Females with risk alleles of variants in CDKAL1 associated with T2DM had a higher risk for poor glycemic control than males.
Figure
Reference
-
1. Lin X, Xu Y, Pan X, Xu J, Ding Y, Sun X, et al. Global, regional, and national burden and trend of diabetes in 195 countries and territories: an analysis from 1990 to 2025. Sci Rep. 2020; 10:14790.
Article2. Jung CH, Son JW, Kang S, Kim WJ, Kim HS, Kim HS, et al. Diabetes fact sheets in Korea, 2020: an appraisal of current status. Diabetes Metab J. 2021; 45:1–10.
Article3. Viana LV, Leitao CB, Kramer CK, Zucatti AT, Jezini DL, Felicio J, et al. Poor glycaemic control in Brazilian patients with type 2 diabetes attending the public healthcare system: a cross-sectional study. BMJ Open. 2013; 3:e003336.
Article4. Lu J, Weng J, Gu W, Guo X, Yang W, Zou D, et al. Non-pharmaceutical factors for poor glycemic control in 13,970 Chinese women with drug-treated type 2 diabetes: a cross-sectional survey in 77 tertiary hospitals in four Chinese cities. Patient Prefer Adherence. 2014; 8:1161–7.5. Aronson R, Orzech N, Ye C, Goldenberg R, Brown V. Specialist-led diabetes registries and predictors of poor glycemic control in type 2 diabetes: insights into the functionally refractory patient from the LMC Diabetes Registry database. J Diabetes. 2016; 8:76–85.
Article6. Haghighatpanah M, Nejad A, Haghighatpanah M, Thunga G, Mallayasamy S. Factors that correlate with poor glycemic control in type 2 diabetes mellitus patients with complications. Osong Public Health Res Perspect. 2018; 9:167–74.
Article7. Kayar Y, Ilhan A, Kayar NB, Unver N, Coban G, Ekinci I, et al. Relationship between the poor glycemic control and risk factors, life style and complications. Biome Res. 2017; 28:1581–6.8. Lima RF, Fontbonne A, Carvalho EM, Montarroyos UR, Barreto MN, Cesse EA. Factors associated with glycemic control in people with diabetes at the Family Health Strategy in Pernambuco. Rev Esc Enferm USP. 2016; 50:937–45.
Article9. Cai X, Hu D, Pan C, Li G, Lu J, Ji Q, et al. The risk factors of glycemic control, blood pressure control, lipid control in Chinese patients with newly diagnosed type 2 diabetes: a nationwide prospective cohort study. Sci Rep. 2019; 9:7709.10. Franzini L, Ardigo D, Cavalot F, Miccoli R, Rivellese AA, Trovati M, et al. Women show worse control of type 2 diabetes and cardiovascular disease risk factors than men: results from the MIND.IT Study Group of the Italian Society of Diabetology. Nutr Metab Cardiovasc Dis. 2013; 23:235–41.
Article11. Al-Azzam SI, Khabour OF, Alzoubi KH, Mukattash TL, Ghanma M, Saleh H. The role of adiponectin gene variants in glycemic control in patients with type 2 diabetes. Endocr Res. 2014; 39:13–7.
Article12. Zhang X, Sun J, Han W, Jiang Y, Peng S, Shan Z, et al. The type 2 deiodinase Thr92Ala polymorphism is associated with worse glycemic control in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. J Diabetes Res. 2016; 2016:5928726.13. Rotroff DM, Yee SW, Zhou K, Marvel SW, Shah HS, Jack JR, et al. Genetic variants in CPA6 and PRPF31 are associated with variation in response to metformin in individuals with type 2 diabetes. Diabetes. 2018; 67:1428–40.14. Cho SB, Jang JH, Chung MG, Kim SC. Exome chip analysis of 14,026 Koreans reveals known and newly discovered genetic loci associated with type 2 diabetes mellitus. Diabetes Metab J. 2021; 45:231–40.
Article15. Kim Y, Han BG; KoGES group. Cohort profile: the Korean Genome and Epidemiology Study (KoGES) consortium. Int J Epidemiol. 2017; 46:e20.
Article16. Jones BL, Nagin DS, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociol Methods Res. 2001; 29:374–93.
Article17. Baumgartner SE, Leydesdorff L. Group-based trajectory modeling (GBTM) of citations in scholarly literature: dynamic qualities of “transient” and “sticky knowledge claims”. J Am Soc Inf Sci Technol. 2014; 65:797–811.
Article18. Andruff H, Carraro N, Thompson A, Gaudreau P, Louvet B. Latent class growth modelling: a tutorial. Tutor Quant Methods Psychol. 2009; 5:11–24.
Article19. Costanzo S, Di Castelnuovo A, Donati MB, Iacoviello L, de Gaetano G. Alcohol consumption and mortality in patients with cardiovascular disease: a meta-analysis. J Am Coll Cardiol. 2010; 55:1339–47.20. Wang T, Huang T, Heianza Y, Sun D, Zheng Y, Ma W, et al. Genetic susceptibility, change in physical activity, and long-term weight gain. Diabetes. 2017; 66:2704–12.
Article21. Knol MJ, VanderWeele TJ. Recommendations for presenting analyses of effect modification and interaction. Int J Epidemiol. 2012; 41:514–20.
Article22. Tuerxunyiming M, Mohemaiti P, Wufuer H, Tuheti A. Association of rs7754840 G/C polymorphisms in CDKAL1 with type 2 diabetes: a meta-analysis of 70141 subjects. Int J Clin Exp Med. 2015; 8:17392–405.23. Deshmukh HA, Madsen AL, Vinuela A, Have CT, Grarup N, Tura A, et al. Genome-wide association analysis of pancreatic beta-cell glucose sensitivity. J Clin Endocrinol Metab. 2021; 106:80–90.
Article24. Ryoo H, Woo J, Kim Y, Lee C. Heterogeneity of genetic associations of CDKAL1 and HHEX with susceptibility of type 2 diabetes mellitus by gender. Eur J Hum Genet. 2011; 19:672–5.
Article25. Klimentidis YC, Lemas DJ, Wiener HH, O’Brien DM, Havel PJ, Stanhope KL, et al. CDKAL1 and HHEX are associated with type 2 diabetes-related traits among Yup’ik people. J Diabetes. 2014; 6:251–9.26. Peng F, Hu D, Gu C, Li X, Li Y, Jia N, et al. The relationship between five widely-evaluated variants in CDKN2A/B and CDKAL1 genes and the risk of type 2 diabetes: a meta-analysis. Gene. 2013; 531:435–43.
Article27. Nfor ON, Wu MF, Lee CT, Wang L, Liu WH, Tantoh DM, et al. Body mass index modulates the association between CDKAL1 rs10946398 variant and type 2 diabetes among Taiwanese women. Sci Rep. 2018; 8:13235.
Article28. Margolis KL, Bonds DE, Rodabough RJ, Tinker L, Phillips LS, Allen C, et al. Effect of oestrogen plus progestin on the incidence of diabetes in postmenopausal women: results from the Women’s Health Initiative Hormone Trial. Diabetologia. 2004; 47:1175–87.
Article29. Schroner Z, Javorsky M, Tkacova R, Klimcakova L, Dobrikova M, Habalova V, et al. Effect of sulphonylurea treatment on glycaemic control is related to TCF7L2 genotype in patients with type 2 diabetes. Diabetes Obes Metab. 2011; 13:89–91.
Article30. Soltani G, Hatefi Z, Salehi AR, Khosravi S, Ghiasi MR, Teke K, et al. Pharmacogenomics of sulfonylureas response in relation to rs7754840 polymorphisms in cyclin-dependent kinase 5 regulatory subunit-associated protein 1-like (CDKAL1) gene in Iranian type 2 diabetes patients. Adv Biomed Res. 2018; 7:96.
Article31. Schroner Z, Javorsky M, Haluskova J, Klimcakova L, Babjakova E, Fabianova M, et al. Variation in CDKAL1 gene is associated with therapeutic response to sulphonylureas. Physiol Res. 2012; 61:177–83.
Article32. ADVANCE Collaborative Group, Patel A, MacMahon S, Chalmers J, Neal B, Billot L, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008; 358:2560–72.
Article33. Liu NJ, Xiong Q, Wu HH, Li YL, Yang Z, Tao XM, et al. The association analysis polymorphism of CDKAL1 and diabetic retinopathy in Chinese Han population. Int J Ophthalmol. 2016; 9:707–12.
Article34. Jialal I, Amess W, Kaur M. Management of hypertriglyceridemia in the diabetic patient. Curr Diab Rep. 2010; 10:316–20.
Article35. Zhao J, Zhang Y, Wei F, Song J, Cao Z, Chen C, et al. Triglyceride is an independent predictor of type 2 diabetes among middle-aged and older adults: a prospective study with 8-year follow-ups in two cohorts. J Transl Med. 2019; 17:403.
Article36. Zheng D, Dou J, Liu G, Pan Y, Yan Y, Liu F, et al. Association between triglyceride level and glycemic control among insulintreated patients with type 2 diabetes. J Clin Endocrinol Metab. 2019; 104:1211–20.
Article37. Ye X, Kong W, Zafar MI, Chen LL. Serum triglycerides as a risk factor for cardiovascular diseases in type 2 diabetes mellitus: a systematic review and meta-analysis of prospective studies. Cardiovasc Diabetol. 2019; 18:48.
Article38. Choi SW, Mak TS, O’Reilly PF. Tutorial: a guide to performing polygenic risk score analyses. Nat Protoc. 2020; 15:2759–72.
Article39. Spracklen CN, Horikoshi M, Kim YJ, Lin K, Bragg F, Moon S, et al. Identification of type 2 diabetes loci in 433,540 East Asian individuals. Nature. 2020; 582:240–5.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Glycemic Control in Diabetic Patients with Diabetic Nephropathy
- Genome-Wide Association Study Identifies Two Novel Loci with Sex-Specific Effects for Type 2 Diabetes Mellitus and Glycemic Traits in a Korean Population
- Recapitulation of previously reported associations for type 2 diabetes and metabolic traits in the 126K East Asians
- Association of CDKAL1 Polymorphisms with Early-Onset Atopic Dermatitis in Koreans
- The association between depressive symptoms and glycemic control in the patients with diabetes mellitus