Diabetes Metab J.
2022 Nov;46(6):855-865. 10.4093/dmj.2021.0264.
A Real-World Study of Long-Term Safety and Efficacy of Lobeglitazone in Korean Patients with Type 2 Diabetes Mellitus
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, Soonchunhyang University Bucheon Hospital, Soonchunhyang University College of Medicine, Bucheon, Korea
- 2Division of Endocrinology and Metabolism, Department of Internal Medicine, Yeouido St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 3Division of Endocrinology and Metabolism, Department of Internal Medicine, Konkuk University School of Medicine, Seoul, Korea
- 4Division of Endocrinology and Metabolism, Department of Internal Medicine, Inje University Ilsan Paik Hospital, College of Medicine, Inje University, Goyang, Korea
- 5Division of Endocrinology and Metabolism, Department of Internal Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea
- 6Division of Endocrinology and Metabolism, Department of Internal Medicine, Soonchunhyang University Seoul Hospital, Soonchunhyang University College of Medicine, Seoul, Korea
- 7Division of Endocrinology and Metabolism, Department of Internal Medicine, Inje University Sanggye Paik Hospital, College of Medicine, Inje University, Seoul, Korea
- 8Division of Endocrinology and Metabolism, Department of Internal Medicine, Hallym University Kangnam Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Korea
- 9Division of Endocrinology and Metabolism, Department of Internal Medicine, Wonju Severance Christian Hospital, Yonsei University Wonju College of Medicine, Wonju, Korea
- 10Division of Endocrinology and Metabolism, Department of Internal Medicine, Myongji Hospital, Hanyang University College of Medicine, Goyang, Korea
- 11Division of Endocrinology and Metabolism, Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
- 12Division of Endocrinology and Metabolism, Department of Internal Medicine, Soonchunhyang University Cheonan Hospital, Soonchunhyang University College of Medicine, Cheonan, Korea
- 13Division of Endocrinology and Metabolism, Department of Internal Medicine, Kyung Hee University Hospital at Gangdong, College of Medicine, Kyung Hee University, Seoul, Korea
- 14Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, Korea
- 15Department of Drug Safety Research, Chong Kun Dang Pharmaceutical Corporation, Seoul, Korea
- 16Division of Endocrinology and Metabolism, Department of Internal Medicine, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2536144
- DOI: http://doi.org/10.4093/dmj.2021.0264
Abstract
- Background
Thiazolidinediones (TZDs) have been associated with various safety concerns including weight gain, bladder cancer, and congestive heart failure (CHF). This study evaluated the efficacy and safety of lobeglitazone, a novel TZD in patients with type 2 diabetes mellitus (T2DM) in real practice.
Methods
In this non-interventional, multi-center, retrospective, and observational study conducted at 15 tertiary or secondary referral hospitals in Korea, a total of 2,228 patients with T2DM who received lobeglitazone 0.5 mg for more than 1 year were enrolled.
Results
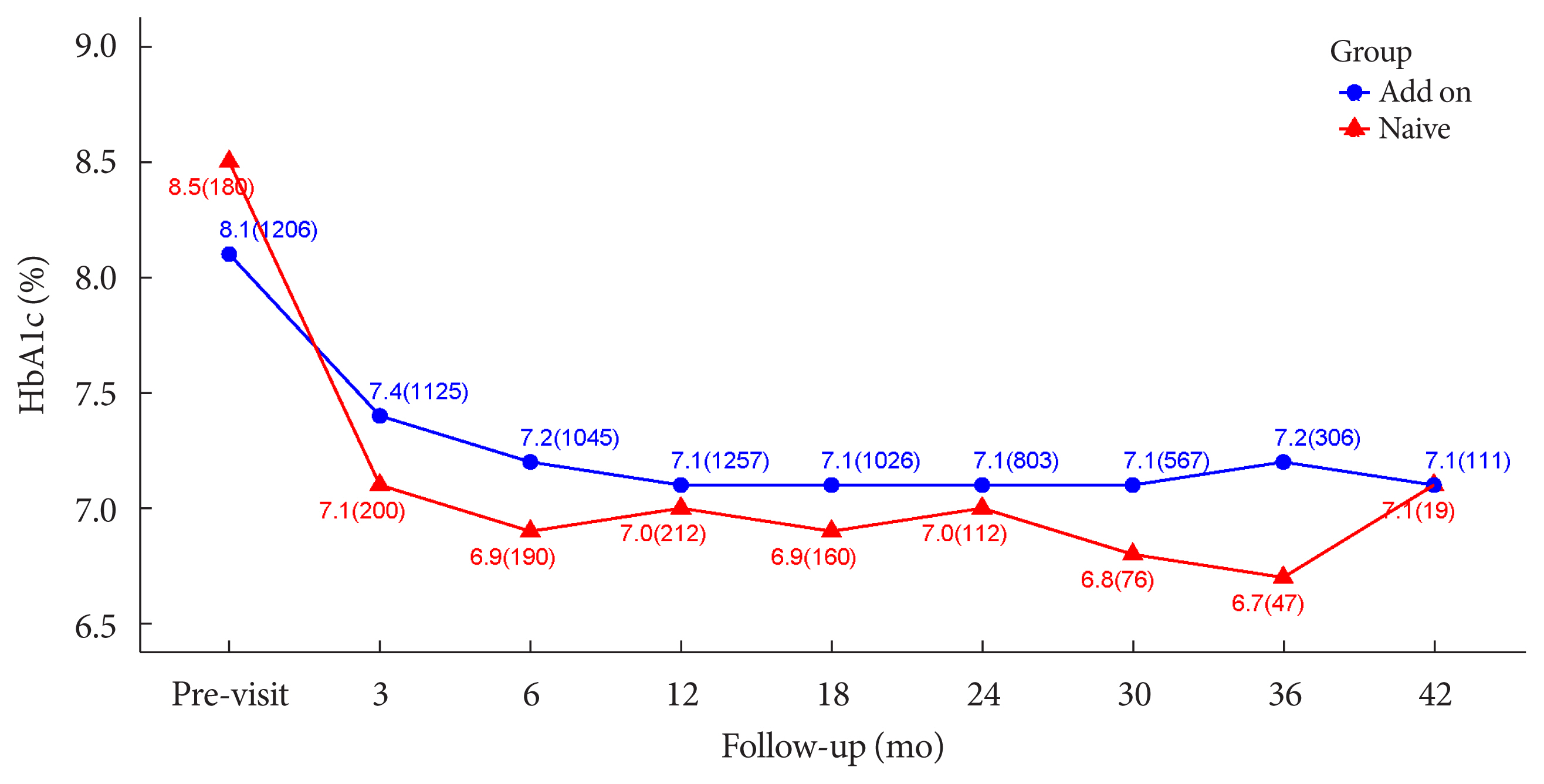
Overall adverse events (AEs) occurred in 381 patients (17.10%) including edema in 1.97% (n=44). Cerebrovascular and cardiovascular diseases were identified in 0.81% (n=18) and 0.81% (n=18), respectively. One case of CHF was reported as an AE. Edema occurred in 1.97% (n=44) of patients. Hypoglycemia occurred in 2.47% (n=55) of patients. Fracture occurred in 1.17% (n=26) of all patients. Lobeglitazone significantly decreased HbA1c level, resulting in a mean treatment difference of -1.05%± 1.35% (P<0.001), and decreased total cholesterol, triglyceride, and low-density lipoprotein cholesterol. However, it increased high-density lipoprotein cholesterol, regardless of statin administration. The patients who received lobeglitazone 0.5 mg showed an apparent reduction in glycosylated hemoglobin (HbA1c) from baseline during the first 6 months of treatment. The HbA1c levels remained stable between months 6 and 42.
Conclusion
Lobeglitazone has long-term safety profile, good glycemic-lowering effect and long-term durability of glycemic control in real-world clinical settings.
Figure
Cited by 1 articles
-
Oldies but Goodies: Thiazolidinedione as an Insulin Sensitizer with Cardioprotection
Eun-Hee Cho
Diabetes Metab J. 2022;46(6):827-828. doi: 10.4093/dmj.2022.0372.
Reference
-
1. Yki-Jarvinen H. Thiazolidinediones. N Engl J Med. 2004; 351:1106–18.
Article2. Kwon MJ, Lee YJ, Jung HS, Shin HM, Kim TN, Lee SH, et al. The direct effect of lobeglitazone, a new thiazolidinedione, on pancreatic beta cells: a comparison with other thiazolidinediones. Diabetes Res Clin Pract. 2019; 151:209–23.
Article3. Xiang AH, Peters RK, Kjos SL, Marroquin A, Goico J, Ochoa C, et al. Effect of pioglitazone on pancreatic beta-cell function and diabetes risk in Hispanic women with prior gestational diabetes. Diabetes. 2006; 55:517–22.4. Yu JG, Javorschi S, Hevener AL, Kruszynska YT, Norman RA, Sinha M, et al. The effect of thiazolidinediones on plasma adiponectin levels in normal, obese, and type 2 diabetic subjects. Diabetes. 2002; 51:2968–74.
Article5. Dandona P, Aljada A, Chaudhuri A. Vascular reactivity and thiazolidinediones. Am J Med. 2003; 115:Suppl 8A. 81S–86S.
Article6. Garg R, Kumbkarni Y, Aljada A, Mohanty P, Ghanim H, Hamouda W, et al. Troglitazone reduces reactive oxygen species generation by leukocytes and lipid peroxidation and improves flow-mediated vasodilatation in obese subjects. Hypertension. 2000; 36:430–5.
Article7. Winkler K, Konrad T, Fullert S, Friedrich I, Destani R, Baumstark MW, et al. Pioglitazone reduces atherogenic dense LDL particles in nondiabetic patients with arterial hypertension: a double-blind, placebo-controlled study. Diabetes Care. 2003; 26:2588–94.8. Kim SG, Kim DM, Woo JT, Jang HC, Chung CH, Ko KS, et al. Efficacy and safety of lobeglitazone monotherapy in patients with type 2 diabetes mellitus over 24-weeks: a multicenter, randomized, double-blind, parallel-group, placebo controlled trial. PLoS One. 2014; 9:e92843.
Article9. Kim SH, Kim SG, Kim DM, Woo JT, Jang HC, Chung CH, et al. Safety and efficacy of lobeglitazone monotherapy in patients with type 2 diabetes mellitus over 52 weeks: an open-label extension study. Diabetes Res Clin Pract. 2015; 110:e27–30.
Article10. Rajagopalan R, Rosenson RS, Fernandes AW, Khan M, Murray FT. Association between congestive heart failure and hospitalization in patients with type 2 diabetes mellitus receiving treatment with insulin or pioglitazone: a retrospective data analysis. Clin Ther. 2004; 26:1400–10.
Article11. Mamtani R, Haynes K, Bilker WB, Vaughn DJ, Strom BL, Glanz K, et al. Association between longer therapy with thiazolidinediones and risk of bladder cancer: a cohort study. J Natl Cancer Inst. 2012; 104:1411–21.
Article12. Kahn SE, Zinman B, Lachin JM, Haffner SM, Herman WH, Holman RR, et al. Rosiglitazone-associated fractures in type 2 diabetes: an analysis from A Diabetes Outcome Progression Trial (ADOPT). Diabetes Care. 2008; 31:845–51.13. Fonseca V. Effect of thiazolidinediones on body weight in patients with diabetes mellitus. Am J Med. 2003; 115:Suppl 8A. 42S–48S.
Article14. Levin D, Bell S, Sund R, Hartikainen SA, Tuomilehto J, Pukkala E, et al. Pioglitazone and bladder cancer risk: a multipopulation pooled, cumulative exposure analysis. Diabetologia. 2015; 58:493–504.
Article15. Tuccori M, Filion KB, Yin H, Yu OH, Platt RW, Azoulay L. Pioglitazone use and risk of bladder cancer: population based cohort study. BMJ. 2016; 352:i1541.
Article16. Nesto RW, Bell D, Bonow RO, Fonseca V, Grundy SM, Horton ES, et al. Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association. Diabetes Care. 2004; 27:256–63.
Article17. Singh S, Loke YK, Furberg CD. Thiazolidinediones and heart failure: a teleo-analysis. Diabetes Care. 2007; 30:2148–53.18. Mamtani R, Haynes K, Bilker WB, Vaughn DJ, Strom BL, Glanz K, et al. Long-term therapy with thiazolidinediones and the risk of bladder cancer: a cohort study. J Clin Oncol. 2012; 30(15 Suppl):1503.
Article19. Filipova E, Uzunova K, Kalinov K, Vekov T. Effects of pioglitazone therapy on blood parameters, weight and BMI: a meta-analysis. Diabetol Metab Syndr. 2017; 9:90.
Article20. Tang WH, Francis GS, Hoogwerf BJ, Young JB. Fluid retention after initiation of thiazolidinedione therapy in diabetic patients with established chronic heart failure. J Am Coll Cardiol. 2003; 41:1394–8.
Article21. Yoshimoto T, Naruse M, Nishikawa M, Naruse K, Tanabe A, Seki T, et al. Antihypertensive and vasculo- and renoprotective effects of pioglitazone in genetically obese diabetic rats. Am J Physiol. 1997; 272(6 Pt 1):E989–96.
Article22. Hosokawa M, Tsukada H, Fukuda K, Oya M, Onomura M, Nakamura H, et al. Troglitazone inhibits bicarbonate secretion in rat and human duodenum. J Pharmacol Exp Ther. 1999; 290:1080–4.23. Walker AB, Naderali EK, Chattington PD, Buckingham RE, Williams G. Differential vasoactive effects of the insulin sensitizers rosiglitazone (BRL 49653) and troglitazone on human small arteries in vitro. Diabetes. 1998; 47:810–4.
Article24. Wan Y. PPARγ in bone homeostasis. Trends Endocrinol Metab. 2010; 21:722–8.
Article25. Patel JJ, Butters OR, Arnett TR. PPAR agonists stimulate adipogenesis at the expense of osteoblast differentiation while inhibiting osteoclast formation and activity. Cell Biochem Funct. 2014; 32:368–77.
Article26. Billington EO, Grey A, Bolland MJ. The effect of thiazolidinediones on bone mineral density and bone turnover: systematic review and meta-analysis. Diabetologia. 2015; 58:2238–46.
Article27. Dormandy J, Bhattacharya M, van Troostenburg de Bruyn AR; PROactive investigators. Safety and tolerability of pioglitazone in high-risk patients with type 2 diabetes: an overview of data from PROactive. Drug Saf. 2009; 32:187–202.
Article28. Lim S, Kim KM, Kim SG, Kim DM, Woo JT, Chung CH, et al. Effects of lobeglitazone, a novel thiazolidinedione, on bone mineral density in patients with type 2 diabetes mellitus over 52 weeks. Diabetes Metab J. 2017; 41:377–85.
Article29. Lee HW, Kim BY, Ahn JB, Kang SK, Lee JH, Shin JS, et al. Molecular design, synthesis, and hypoglycemic and hypolipidemic activities of novel pyrimidine derivatives having thiazolidinedione. Eur J Med Chem. 2005; 40:862–74.
Article30. Lim S, Lee KS, Lee JE, Park HS, Kim KM, Moon JH, et al. Effect of a new PPAR-gamma agonist, lobeglitazone, on neointimal formation after balloon injury in rats and the development of atherosclerosis. Atherosclerosis. 2015; 243:107–19.
Article31. Kim KM, Jin HJ, Lee SY, Maeng HJ, Lee GY, Oh TJ, et al. Effects of Lobeglitazone, a new thiazolidinedione, on osteoblastogenesis and bone mineral density in mice. Endocrinol Metab (Seoul). 2017; 32:389–95.
Article32. Lee HS, Chang M, Lee JE, Kim W, Hwang IC, Kim DH, et al. Carcinogenicity study of CKD-501, a novel dual peroxisome proliferator-activated receptors α and γ agonist, following oral administration to Sprague Dawley rats for 94–101 weeks. Regul Toxicol Pharmacol. 2014; 69:207–16.33. Moon KS, Lee JE, Lee HS, Hwang IC, Kim DH, Park HK, et al. CKD-501, a novel selective PPARγ agonist, shows no carcinogenic potential in ICR mice following oral administration for 104 weeks. J Appl Toxicol. 2014; 34:1271–84.
Article34. Bae J, Park T, Kim H, Lee M, Cha BS. Lobeglitazone: a novel thiazolidinedione for the management of type 2 diabetes mellitus. Diabetes Metab J. 2021; 45:326–36.
Article35. Kim JW, Kim JR, Yi S, Shin KH, Shin HS, Yoon SH, et al. Tolerability and pharmacokinetics of lobeglitazone (CKD-501), a peroxisome proliferator-activated receptor-γ agonist: a single- and multiple-dose, double-blind, randomized control study in healthy male Korean subjects. Clin Ther. 2011; 33:1819–30.
Article36. Mamza J, Mehta R, Donnelly R, Idris I. Important differences in the durability of glycaemic response among second-line treatment options when added to metformin in type 2 diabetes: a retrospective cohort study. Ann Med. 2016; 48:224–34.
Article37. Lee MA, Tan L, Yang H, Im YG, Im YJ. Structures of PPARγ complexed with lobeglitazone and pioglitazone reveal key determinants for the recognition of antidiabetic drugs. Sci Rep. 2017; 7:16837.
Article38. Jang JY, Bae H, Lee YJ, Choi YI, Kim HJ, Park SB, et al. Structural basis for the enhanced anti-diabetic efficacy of lobeglitazone on PPARγ. Sci Rep. 2018; 8:31.
Article39. Jin SM, Park CY, Cho YM, Ku BJ, Ahn CW, Cha BS, et al. Lobeglitazone and pioglitazone as add-ons to metformin for patients with type 2 diabetes: a 24-week, multicentre, randomized, double-blind, parallel-group, active-controlled, phase III clinical trial with a 28-week extension. Diabetes Obes Metab. 2015; 17:599–602.
Article40. Ha KH, Park CY, Jeong IK, Kim HJ, Kim SY, Kim WJ, et al. Clinical characteristics of people with newly diagnosed type 2 diabetes between 2015 and 2016: difference by age and body mass index. Diabetes Metab J. 2018; 42:137–46.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Lobeglitazone: A Novel Thiazolidinedione for the Management of Type 2 Diabetes Mellitus
- Long-term efficacy and safety of tofacitinib in patients with ulcerative colitis: 3-year results from a real-world study
- Clinical Efficacy of Sodium-Glucose Cotransporter 2 Inhibitor and Glucagon-Like Peptide-1 Receptor Agonist Combination Therapy in Type 2 Diabetes Mellitus: Real-World Study (Diabetes Metab J 2022;46: 658-62)
- Clinical Efficacy of Sodium-Glucose Cotransporter 2 Inhibitor and Glucagon-Like Peptide-1 Receptor Agonist Combination Therapy in Type 2 Diabetes Mellitus: Real-World Study
- Effect of Dapagliflozin in Combination with Lobeglitazone and Metformin in Korean Patients with Type 2 Diabetes in Real-World Clinical Practice