Lobeglitazone: A Novel Thiazolidinedione for the Management of Type 2 Diabetes Mellitus
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, International St. Mary’s Hospital, Catholic Kwandong University College of Medicine, Incheon, Korea
- 2Department of Clinical Research Design and Evaluation, Samsung Advanced Institute for Health Sciences & Technology, Sungkyunkwan University, Seoul, Korea
- 3Medical information and Pharmacovigilance Team, CKD Pharmaceutical Corp., Seoul, Korea
- 4Division of Endocrinology and Metabolism, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea
- KMID: 2516334
- DOI: http://doi.org/10.4093/dmj.2020.0272
Abstract
- Type 2 diabetes mellitus (T2DM) is characterized by insulin resistance and β-cell dysfunction. Among available oral antidiabetic agents, only the thiazolidinediones (TZDs) primarily target insulin resistance. TZDs improve insulin sensitivity by activating peroxisome proliferator-activated receptor γ. Rosiglitazone and pioglitazone have been used widely for T2DM treatment due to their potent glycemic efficacy and low risk of hypoglycemia. However, their use has decreased because of side effects and safety issues, such as cardiovascular concerns and bladder cancer. Lobeglitazone (Chong Kun Dang Pharmaceutical Corporation), a novel TZD, was developed to meet the demands for an effective and safe TZD. Lobeglitazone shows similar glycemic efficacy to pioglitazone, with a lower effective dose, and favorable safety results. It also showed pleiotropic effects in preclinical and clinical studies. In this article, we summarize the pharmacologic, pharmacokinetic, and clinical characteristics of lobeglitazone.
Figure
Cited by 2 articles
-
Effect of the addition of thiazolidinedione to sodium-glucose cotransporter 2 inhibitor therapy on lipid levels in type 2 diabetes mellitus: a retrospective study using Korean National Health Insurance Service data
Taegyun Park, Kyungdo Han, Dongwook Shin, Jongho Park
Cardiovasc Prev Pharmacother. 2022;4(3):114-122. doi: 10.36011/cpp.2022.4.e15.A Real-World Study of Long-Term Safety and Efficacy of Lobeglitazone in Korean Patients with Type 2 Diabetes Mellitus
Bo-Yeon Kim, Hyuk-Sang Kwon, Suk Kyeong Kim, Jung-Hyun Noh, Cheol-Young Park, Hyeong-Kyu Park, Kee-Ho Song, Jong Chul Won, Jae Myung Yu, Mi Young Lee, Jae Hyuk Lee, Soo Lim, Sung Wan Chun, In-Kyung Jeong, Choon Hee Chung, Seung Jin Han, Hee-Seok Kim, Ju-Young Min, Sungrae Kim
Diabetes Metab J. 2022;46(6):855-865. doi: 10.4093/dmj.2021.0264.
Reference
-
1. Kahn SE. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia. 2003; 46:3–19.
Article2. Lebovitz HE. Thiazolidinediones: the forgotten diabetes medications. Curr Diab Rep. 2019; 19:151.
Article3. Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma). J Biol Chem. 1995; 270:12953–6.4. Kohlroser J, Mathai J, Reichheld J, Banner BF, Bonkovsky HL. Hepatotoxicity due to troglitazone: report of two cases and review of adverse events reported to the United States Food and Drug Administration. Am J Gastroenterol. 2000; 95:272–6.
Article5. Hanefeld M, Pfutzner A, Forst T, Lubben G. Glycemic control and treatment failure with pioglitazone versus glibenclamide in type 2 diabetes mellitus: a 42-month, open-label, observational, primary care study. Curr Med Res Opin. 2006; 22:1211–5.
Article6. Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006; 355:2427–43.
Article7. Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007; 356:2457–71.
Article8. Soccio RE, Chen ER, Lazar MA. Thiazolidinediones and the promise of insulin sensitization in type 2 diabetes. Cell Metab. 2014; 20:573–91.
Article9. Lee MA, Tan L, Yang H, Im YG, Im YJ. Structures of PPARγ complexed with lobeglitazone and pioglitazone reveal key determinants for the recognition of antidiabetic drugs. Sci Rep. 2017; 7:16837.
Article10. Jang JY, Bae H, Lee YJ, Choi YI, Kim HJ, Park SB, et al. Structural basis for the enhanced anti-diabetic efficacy of lobeglitazone on PPARγ. Sci Rep. 2018; 8:31.
Article11. Chong Kun Dang Pharmaceutical Corp.: Data on file, 2013. Available from: http://www.ckdpharm.com (cited 2021 Mar 20).12. Kwon MJ, Lee YJ, Jung HS, Shin HM, Kim TN, Lee SH, et al. The direct effect of lobeglitazone, a new thiazolidinedione, on pancreatic beta cells: a comparison with other thiazolidinediones. Diabetes Res Clin Pract. 2019; 151:209–23.
Article13. Kim G, Lee YH, Yun MR, Lee JY, Shin EG, Lee BW, et al. Effects of lobeglitazone, a novel thiazolidinedione, on adipose tissue remodeling and brown and beige adipose tissue development in db/db mice. Int J Obes (Lond). 2018; 42:542–51.
Article14. Rocha RF, Rodrigues T, Menegatti ACO, Bernardes GJL, Terenzi H. The antidiabetic drug lobeglitazone has the potential to inhibit PTP1B activity. Bioorg Chem. 2020; 100:103927.
Article15. Sohn JH, Kim JI, Jeon YG, Park J, Kim JB. Effects of three thiazolidinediones on metabolic regulation and cold-induced thermogenesis. Mol Cells. 2018; 41:900–8.16. Lee YS, Park JS, Lee DH, Lee DK, Kwon SW, Lee BW, et al. The antidiabetic drug lobeglitazone protects mice from lipogenesisinduced liver injury via mechanistic target of rapamycin complex 1 inhibition. Front Endocrinol (Lausanne). 2018; 9:539.
Article17. Choung S, Joung KH, You BR, Park SK, Kim HJ, Ku BJ. Treatment with lobeglitazone attenuates hepatic steatosis in diet-induced obese mice. PPAR Res. 2018; 2018:4292509.
Article18. Lim S, Lee KS, Lee JE, Park HS, Kim KM, Moon JH, et al. Effect of a new PPAR-gamma agonist, lobeglitazone, on neointimal formation after balloon injury in rats and the development of atherosclerosis. Atherosclerosis. 2015; 243:107–19.
Article19. Choi JY, Ryu J, Kim HJ, Song JW, Jeon JH, Lee DH, et al. Therapeutic effects of targeted PPARγ activation on inflamed highrisk plaques assessed by serial optical imaging in vivo. Theranostics. 2018; 8:45–60.20. Law RE, Goetze S, Xi XP, Jackson S, Kawano Y, Demer L, et al. Expression and function of PPARgamma in rat and human vascular smooth muscle cells. Circulation. 2000; 101:1311–8.21. Jiang C, Ting AT, Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998; 391:82–6.22. Park S, Ahn JW, Jo Y, Kang HY, Kim HJ, Cheon Y, et al. Label-free tomographic imaging of lipid droplets in foam cells for machine-learning-assisted therapeutic evaluation of targeted nanodrugs. ACS Nano. 2020; 14:1856–65.
Article23. Shin NR, Park SH, Ko JW, Cho YK, Lee IC, Kim JC, et al. Lobeglitazone attenuates airway inflammation and mucus hypersecretion in a murine model of ovalbumin-induced asthma. Front Pharmacol. 2018; 9:906.
Article24. Bae KH, Seo JB, Jung YA, Seo HY, Kang SH, Jeon HJ, et al. Lobeglitazone, a novel peroxisome proliferator-activated receptor γ agonist, attenuates renal fibrosis caused by unilateral ureteral obstruction in mice. Endocrinol Metab (Seoul). 2017; 32:115–23.
Article25. Kim KM, Jin HJ, Lee SY, Maeng HJ, Lee GY, Oh TJ, et al. Effects of lobeglitazone, a new thiazolidinedione, on osteoblastogenesis and bone mineral density in mice. Endocrinol Metab (Seoul). 2017; 32:389–95.
Article26. Ali AA, Weinstein RS, Stewart SA, Parfitt AM, Manolagas SC, Jilka RL. Rosiglitazone causes bone loss in mice by suppressing osteoblast differentiation and bone formation. Endocrinology. 2005; 146:1226–35.
Article27. Bilezikian JP, Josse RG, Eastell R, Lewiecki EM, Miller CG, Wooddell M, et al. Rosiglitazone decreases bone mineral density and increases bone turnover in postmenopausal women with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2013; 98:1519–28.
Article28. Tseng CH, Tseng FH. Peroxisome proliferator-activated receptor agonists and bladder cancer: lessons from animal studies. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2012; 30:368–402.
Article29. Yan H, Xie H, Ying Y, Li J, Wang X, Xu X, et al. Pioglitazone use in patients with diabetes and risk of bladder cancer: a systematic review and meta-analysis. Cancer Manag Res. 2018; 10:1627–38.
Article30. Lee HS, Chang M, Lee JE, Kim W, Hwang IC, Kim DH, et al. Carcinogenicity study of CKD-501, a novel dual peroxisome proliferator-activated receptors α and γ agonist, following oral administration to Sprague Dawley rats for 94-101 weeks. Regul Toxicol Pharmacol. 2014; 69:207–16.31. Moon KS, Lee JE, Lee HS, Hwang IC, Kim DH, Park HK, et al. CKD-501, a novel selective PPARγ agonist, shows no carcinogenic potential in ICR mice following oral administration for 104 weeks. J Appl Toxicol. 2014; 34:1271–84.
Article32. Kim JW, Kim JR, Yi S, Shin KH, Shin HS, Yoon SH, et al. Tolerability and pharmacokinetics of lobeglitazone (CKD-501), a peroxisome proliferator-activated receptor-γ agonist: a singleand multiple-dose, double-blind, randomized control study in healthy male Korean subjects. Clin Ther. 2011; 33:1819–30.33. Park MK, Kim TE, Kim J, Kim C, Yoon SH, Cho JY, et al. Tolerability and pharmacokinetics of lobeglitazone, a novel peroxisome proliferator-activated receptor-γ agonist, after a single oral administration in healthy female subjects. Clin Drug Investig. 2014; 34:467–74.
Article34. Kim SY, Jeon JY, Park SJ, Kim MG. Pharmacokinetics of a lobeglitazone/metformin fixed-dose combination tablet (CKD-395 0.5/1000 mg) versus concomitant administration of single agents and the effect of food on the metabolism of CKD-395 in healthy male subjects. Clin Pharmacol Drug Dev. 2019; 8:576–84.35. Shin D, Kim TE, Yoon SH, Cho JY, Shin SG, Jang IJ, et al. Assessment of the pharmacokinetics of co-administered metformin and lobeglitazone, a thiazolidinedione antihyperglycemic agent, in healthy subjects. Curr Med Res Opin. 2012; 28:1213–20.
Article36. Jang K, Jeon JY, Moon SJ, Kim MG. Evaluation of the pharmacokinetic interaction between lobeglitazone and dapagliflozin at steady state. Clin Ther. 2020; 42:295–304.
Article37. Moon SJ, Yu KS, Kim MG. An assessment of pharmacokinetic interaction between lobeglitazone and sitagliptin after multiple oral administrations in healthy men. Clin Ther. 2020; 42:1047–57.
Article38. Kim CO, Sil Oh E, Kim C, Park MS. Pharmacokinetic interaction between amlodipine and lobeglitazone, a novel peroxisome proliferator-activated receptor-γ agonist, in healthy subjects. Clin Ther. 2015; 37:1999–2006.
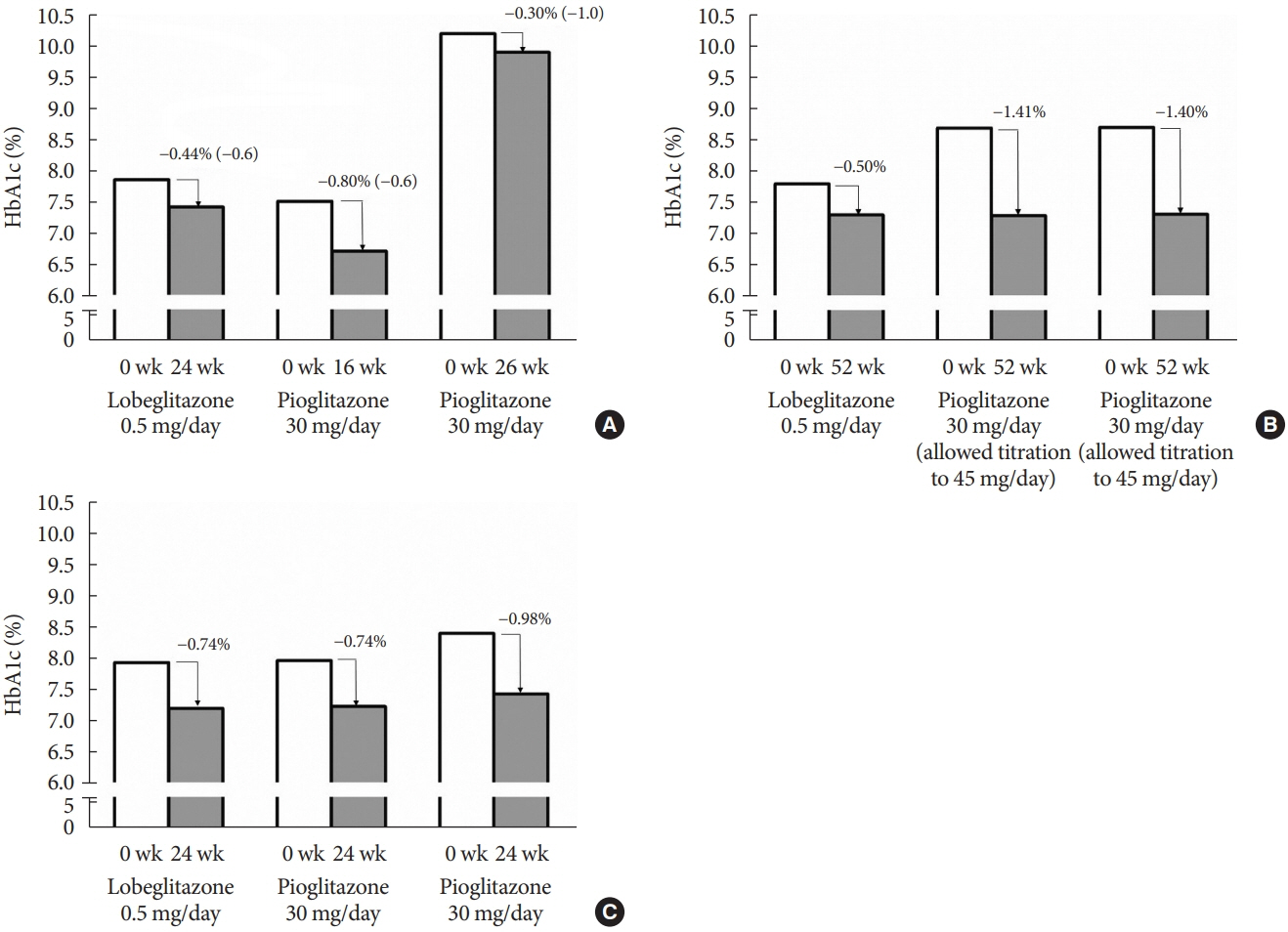
Article39. Jung JA, Lee SY, Kim TE, Kim JR, Kim C, Huh W, et al. Lack of the effect of lobeglitazone, a peroxisome proliferator-activated receptor-γ agonist, on the pharmacokinetics and pharmacodynamics of warfarin. Drug Des Devel Ther. 2015; 9:737–43.40. Oh ES, Kim CO, Kim KH, Kim YN, Kim C, Lee JI, et al. Effect of ketoconazole on lobeglitazone pharmacokinetics in Korean volunteers. Clin Ther. 2014; 36:1064–71.41. Kim SG, Kim DM, Woo JT, Jang HC, Chung CH, Ko KS, et al. Efficacy and safety of lobeglitazone monotherapy in patients with type 2 diabetes mellitus over 24-weeks: a multicenter, randomized, double-blind, parallel-group, placebo controlled trial. PLoS One. 2014; 9:e92843.
Article42. Kim SH, Kim SG, Kim DM, Woo JT, Jang HC, Chung CH, et al. Safety and efficacy of lobeglitazone monotherapy in patients with type 2 diabetes mellitus over 52 weeks: an open-label extension study. Diabetes Res Clin Pract. 2015; 110:e27–30.
Article43. Jin SM, Park CY, Cho YM, Ku BJ, Ahn CW, Cha BS, et al. Lobeglitazone and pioglitazone as add-ons to metformin for patients with type 2 diabetes: a 24-week, multicentre, randomized, double-blind, parallel-group, active-controlled, phase III clinical trial with a 28-week extension. Diabetes Obes Metab. 2015; 17:599–602.44. Kim SG, Kim KJ, Yoon KH, Chun SW, Park KS, Choi KM, et al. Efficacy and safety of lobeglitazone versus sitagliptin as an add-on to metformin in patients with type 2 diabetes with two or more components of metabolic syndrome over 24weeks. Diabetes Obes Metab. 2020; 22:1869–73.
Article45. Lee JY, Cho Y, Lee M, Lee YH, Lee BW, Kang ES, et al. Clinical efficacy of the novel thiazolidinedione lobeglitazone in patients with type 2 diabetes. Diabetes Metab. 2018; 44:452–5.
Article46. Lim S, Ku EJ, Lee SY, Lee JH, Lee JE, Kim KM, et al. Therapeutic efficacy and safety of initial triple combination of metformin, sitagliptin, and lobeglitazone in drug-naïve patients with type 2 diabetes: initial triple study. BMJ Open Diabetes Res Care. 2020; 8:e000807.
Article47. Aronoff S, Rosenblatt S, Braithwaite S, Egan JW, Mathisen AL, Schneider RL. Pioglitazone hydrochloride monotherapy improves glycemic control in the treatment of patients with type 2 diabetes: a 6-month randomized placebo-controlled dose-response study. The Pioglitazone 001 Study Group. Diabetes Care. 2000; 23:1605–11.
Article48. Herz M, Johns D, Reviriego J, Grossman LD, Godin C, Duran S, et al. A randomized, double-blind, placebo-controlled, clinical trial of the effects of pioglitazone on glycemic control and dyslipidemia in oral antihyperglycemic medication-naïve patients with type 2 diabetes mellitus. Clin Ther. 2003; 25:1074–95.49. Schernthaner G, Matthews DR, Charbonnel B, Hanefeld M, Brunetti P; Quartet [corrected] Study Group. Efficacy and safety of pioglitazone versus metformin in patients with type 2 diabetes mellitus: a double-blind, randomized trial. J Clin Endocrinol Metab. 2004; 89:6068–76.50. Charbonnel BH, Matthews DR, Schernthaner G, Hanefeld M, Brunetti P; QUARTET Study Group. A long-term comparison of pioglitazone and gliclazide in patients with type 2 diabetes mellitus: a randomized, double-blind, parallel-group comparison trial. Diabet Med. 2005; 22:399–405.51. Bolli G, Dotta F, Rochotte E, Cohen SE. Efficacy and tolerability of vildagliptin vs. pioglitazone when added to metformin: a 24-week, randomized, double-blind study. Diabetes Obes Metab. 2008; 10:82–90.
Article52. Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012; 142:1592–609.
Article53. Lee YH, Kim JH, Kim SR, Jin HY, Rhee EJ, Cho YM, et al. Lobeglitazone, a novel thiazolidinedione, improves non-alcoholic fatty liver disease in type 2 diabetes: its efficacy and predictive factors related to responsiveness. J Korean Med Sci. 2017; 32:60–9.
Article54. Rosenblatt S, Miskin B, Glazer NB, Prince MJ, Robertson KE; Pioglitazone 026 Study Group. The impact of pioglitazone on glycemic control and atherogenic dyslipidemia in patients with type 2 diabetes mellitus. Coron Artery Dis. 2001; 12:413–23.
Article55. Goke B; German Pioglitazone Study Group. Improved glycemic control and lipid profile in a randomized study of pioglitazone compared with acarbose in patients with type 2 diabetes mellitus. Treat Endocrinol. 2002; 1:329–36.56. Tan M, Johns D, Gonzalez Galvez G, Antunez O, Fabian G, Flores-Lozano F, et al. Effects of pioglitazone and glimepiride on glycemic control and insulin sensitivity in Mexican patients with type 2 diabetes mellitus: a multicenter, randomized, double-blind, parallel-group trial. Clin Ther. 2004; 26:680–93.
Article57. Waugh J, Keating GM, Plosker GL, Easthope S, Robinson DM. Pioglitazone: a review of its use in type 2 diabetes mellitus. Drugs. 2006; 66:85–109.58. Lim S, Kim KM, Kim SG, Kim DM, Woo JT, Chung CH, et al. Effects of lobeglitazone, a novel thiazolidinedione, on bone mineral density in patients with type 2 diabetes mellitus over 52 weeks. Diabetes Metab J. 2017; 41:377–85.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Lobeglitazone, a New Thiazolidinedione, on Osteoblastogenesis and Bone Mineral Density in Mice
- Effects of Lobeglitazone, a Novel Thiazolidinedione, on Bone Mineral Density in Patients with Type 2 Diabetes Mellitus over 52 Weeks
- Lobeglitazone, a Novel Thiazolidinedione, Improves Non-Alcoholic Fatty Liver Disease in Type 2 Diabetes: Its Efficacy and Predictive Factors Related to Responsiveness
- Comprehensive Management in Patients with Type 2 Diabetes Mellitus
- Recent Perspective on Thiazolidinedione