Anesth Pain Med.
2022 Oct;17(4):371-380. 10.17085/apm.22189.
Postoperative mortality in patients with end-stage renal disease according to the use of sugammadex: a single-center retrospective propensity score matched study
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Soonchunhyang University Hospital Seoul, Seoul, Korea
- 2Department of General Surgery, Soonchunhyang University Hospital Seoul, Seoul, Korea
- 3Department of Biostatistics, Soonchunhyang University College of Medicine, Seoul, Korea
- KMID: 2535335
- DOI: http://doi.org/10.17085/apm.22189
Abstract
- excretion. There are restrictions on the use of sugammadex in patients with severe renal impairment. A paucity of data supports the clinical safety of sugammadex in patients with renal impairment. We analyzed mortality after using sugammadex in patients with end-stage renal disease to establish evidence of safety for sugammadex.
Methods
We retrospectively collected the medical records of 2,134 patients with end-stage renal disease who were dependent on hemodialysis and underwent surgery under general anesthesia between January 2018 and December 2019. Propensity score matching was used. The primary outcome was the 30-day mortality rate, and secondary outcomes were the 1-year mortality rate and causes of death.
Results
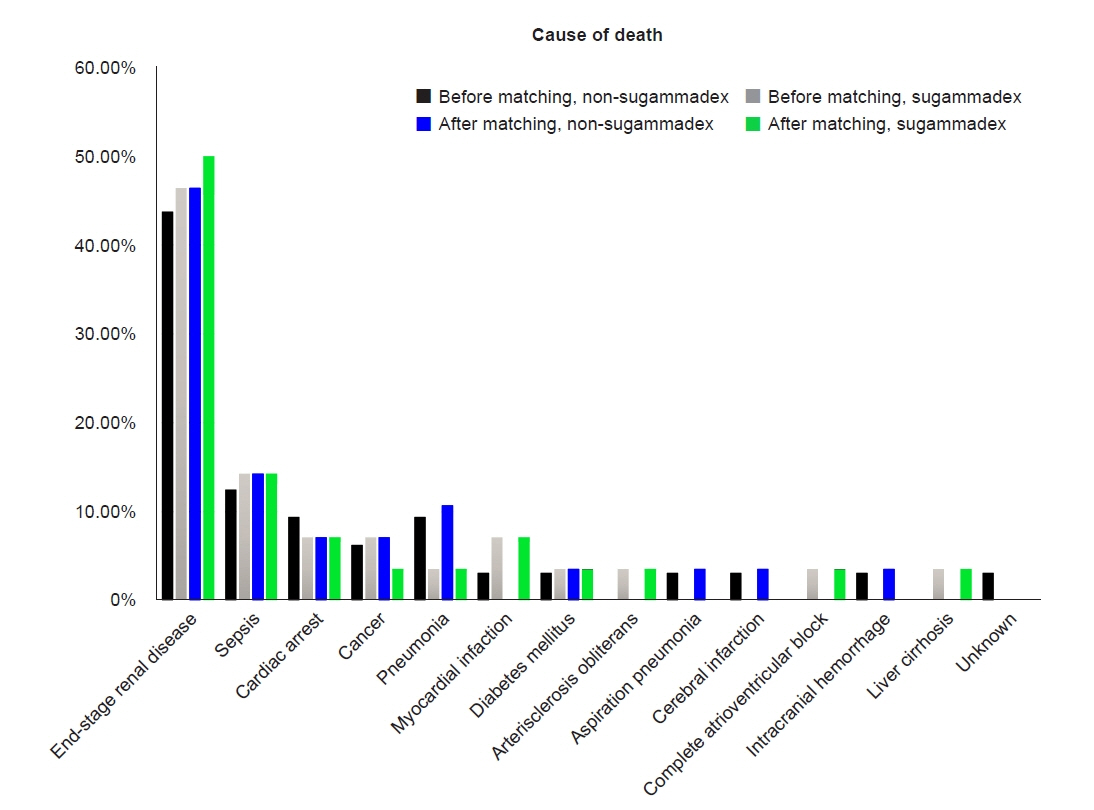
A total of 2,039 patients were included in the study. Sugammadex was administered as a reversal agent for rocuronium in 806 (39.5%) patients; the remaining 1,233 (60.5%) patients did not receive sugammadex. After matching, 1,594 patients were analyzed; 28 (3.5%) of the 797 patients administered sugammadex, and 28 (3.5%) of the 797 patients without sugammadex, died within 30 days after surgery (P > 0.99); 38 (4.8%) of the 797 patients administered sugammadex, and 45 (5.7%) of the 797 patients without sugammadex, died within 1 year after surgery (P = 0.499). No significant differences in the causes of 30-day mortality were observed between the two groups after matching (P = 0.860).
Conclusions
In this retrospective study, sugammadex did not increase the 30-day and 1-year mortality rate after surgery in end-stage renal disease patients.
Figure
Cited by 1 articles
-
Sugammadex administration in patients with end-stage renal disease: a narrative review with recommendations
Seok Kyeong Oh, Byung Gun Lim
Anesth Pain Med. 2023;18(1):11-20. doi: 10.17085/apm.22259.
Reference
-
1. Bom A, Hope F, Rutherford S, Thomson K. Preclinical pharmacology of sugammadex. J Crit Care. 2009; 24:29–35.
Article2. Gijsenbergh F, Ramael S, Houwing N, van Iersel T. First human exposure of Org 25969, a novel agent to reverse the action of rocuronium bromide. Anesthesiology. 2005; 103:695–703.
Article3. Bom A, Bradley M, Cameron K, Clark JK, Van Egmond J, Feilden H, et al. A novel concept of reversing neuromuscular block: chemical encapsulation of rocuronium bromide by a cyclodextrin-based synthetic host. Angew Chem Int Ed Engl. 2002; 41:266–70.
Article4. Butterworth JF, Mackey DC, Wasnick JD. Morgan & Mikhail's clinical anesthesiology. 6th ed. New York (NY): McGraw-Hill;2018. p. 226–7.5. Peeters P, Passier P, Smeets J, Zwiers A, de Zwart M, van de Wetering-Krebbers S, et al. Sugammadex is cleared rapidly and primarily unchanged via renal excretion. Biopharm Drug Dispos. 2011; 32:159–67.
Article6. Staals LM, Snoeck MM, Driessen JJ, van Hamersvelt HW, Flockton EA, van den Heuvel MW, et al. Reduced clearance of rocuronium and sugammadex in patients with severe to end-stage renal failure: a pharmacokinetic study. Br J Anaesth. 2010; 104:31–9.7. Merck Sharp & Dohme. BRIDION®(sugammadex) injection, for intravenous use initial U.S. approval: 2015. U.S. Food and Drug Administration [Internet]. 2015 Dec 15 [updated 2016 Feb 16; cited 2020 Jul 15]. Available from https://www.fda.gov/drugs/drug-approvals-and-databases/drug-trial-snapshot-bridion.8. Cammu G, Van Vlem B, van den Heuvel M, Stet L, el Galta R, Eloot S, et al. Dialysability of sugammadex and its complex with rocuronium in intensive care patients with severe renal impairment. Br J Anaesth. 2012; 109:382–90.
Article9. Grassmann A, Gioberge S, Moeller S, Brown G. End-stage renal disease: global demographics in 2005 and observed trends. Artif Organs. 2006; 30:895–7.10. de Souza CM, Tardelli MA, Tedesco H, Garcia NN, Caparros MP, Alvarez-Gomez JA, et al. Efficacy and safety of sugammadex in the reversal of deep neuromuscular blockade induced by rocuronium in patients with end-stage renal disease: a comparative prospective clinical trial. Eur J Anaesthesiol. 2015; 32:681–6.11. Panhuizen IF, Gold SJ, Buerkle C, Snoeck MM, Harper NJ, Kaspers MJ, et al. Efficacy, safety and pharmacokinetics of sugammadex 4 mg kg-1 for reversal of deep neuromuscular blockade in patients with severe renal impairment. Br J Anaesth. 2015; 114:777–84.
Article12. Staals LM, Snoeck MM, Driessen JJ, Flockton EA, Heeringa M, Hunter JM. Multicentre, parallel-group, comparative trial evaluating the efficacy and safety of sugammadex in patients with end-stage renal failure or normal renal function. Br J Anaesth. 2008; 101:492–7.
Article13. Min KC, Lasseter KC, Marbury TC, Wrishko RE, Hanley WD, Wolford DG, et al. Pharmacokinetics of sugammadex in subjects with moderate and severe renal impairment. Int J Clin Pharmacol Ther. 2017; 55:746–52.
Article14. Adams DR, Tollinche LE, Yeoh CB, Artman J, Mehta M, Phillips D, et al. Short-term safety and effectiveness of sugammadex for surgical patients with end-stage renal disease: a two-centre retrospective study. Anaesthesia. 2020; 75:348–52.
Article15. Paredes S, Porter SB, Porter IE 2nd, Renew JR. Sugammadex use in patients with end-stage renal disease: a historical cohort study. Can J Anaesth. 2020; 67:1789–97.
Article16. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007; 147:573–7.
Article17. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011; 46:399–424.
Article18. Rexsoft. Rex: excel-based statistical analysis software. Seoul: Rexsoft;2018.19. Li G, Freundlich RE, Gupta RK, Hayhurst CJ, Le CH, Martin BJ, et al. Postoperative pulmonary complications' association with sugammadex versus neostigmine: a retrospective registry analysis. Anesthesiology. 2021; 134:862–73.20. Kirmeier E, Eriksson LI, Lewald H, Jonsson Fagerlund M, Hoeft A, Hollmann M, et al. POPULAR Contributors. Post-anaesthesia pulmonary complications after use of muscle relaxants (POPULAR): a multicentre, prospective observational study. Lancet Respir Med 2019; 7: 129-40. Erratum in:. Lancet Respir Med. 2019; 7:e9.21. Kheterpal S, Vaughn MT, Dubovoy TZ, Shah NJ, Bash LD, Colquhoun DA, et al. Sugammadex versus neostigmine for reversal of neuromuscular blockade and postoperative pulmonary complications (STRONGER): a multicenter matched cohort analysis. Anesthesiology. 2020; 132:1371–81.22. Ledowski T, Falke L, Johnston F, Gillies E, Greenaway M, De Mel A, et al. Retrospective investigation of postoperative outcome after reversal of residual neuromuscular blockade: sugammadex, neostigmine or no reversal. Eur J Anaesthesiol. 2014; 31:423–9.23. Blobner M, Hunter JM, Meistelman C, Hoeft A, Hollmann MW, Kirmeier E, et al. Use of a train-of-four ratio of 0.95 versus 0.9 for tracheal extubation: an exploratory analysis of POPULAR data. Br J Anaesth. 2020; 124:63–72.
Article24. Sacan O, White PF, Tufanogullari B, Klein K. Sugammadex reversal of rocuronium-induced neuromuscular blockade: a comparison with neostigmine-glycopyrrolate and edrophonium-atropine. Anesth Analg. 2007; 104:569–74.
Article25. Hristovska AM, Duch P, Allingstrup M, Afshari A. The comparative efficacy and safety of sugammadex and neostigmine in reversing neuromuscular blockade in adults. A Cochrane systematic review with meta-analysis and trial sequential analysis. Anaesthesia. 2018; 73:631–41.
Article26. Mathew A, Devereaux PJ, O'Hare A, Tonelli M, Thiessen-Philbrook H, Nevis IF, et al. Chronic kidney disease and postoperative mortality: a systematic review and meta-analysis. Kidney Int. 2008; 73:1069–81.
Article27. Saran R, Robinson B, Shahinian V. 2018 USRDS annual data report: executive summary. Bethesda (MD): United States Renal Data System;2018.28. Ganesh SK, Stack AG, Levin NW, Hulbert-Shearon T, Port FK. Association of elevated serum PO4, Ca x PO4 product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J Am Soc Nephrol. 2001; 12:2131–8.29. Bradbury BD, Fissell RB, Albert JM, Anthony MS, Critchlow CW, Pisoni RL, et al. Predictors of early mortality among incident US hemodialysis patients in the dialysis outcomes and practice patterns study (DOPPS). Clin J Am Soc Nephrol. 2007; 2:89–99.
Article30. Bae MH, Lee JH, Yang DH, Park HS, Cho Y, Chae SC. Usefulness of surgical parameters as predictors of postoperative cardiac events in patients undergoing non-cardiac surgery. Circ J. 2014; 78:718–23.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Sugammadex administration in patients with end-stage renal disease: a narrative review with recommendations
- Initial experience with the unrestricted introduction of sugammadex at a large academic medical center: a retrospective observational study examining postoperative mechanical ventilation and efficiency outcomes
- Comparison of postoperative nausea and vomiting between remimazolam and propofol: a propensity score-matched, retrospective, observational, single-center cohort study
- Outcome of Early Initiation of Peritoneal Dialysis in Patients with End-Stage Renal Failure
- Effect of albumin on the outcomes in septic patients with hypoalbuminemia in the emergency department: a propensity score-matched retrospective cohort study