Anesth Pain Med.
2022 Jul;17(3):262-270. 10.17085/apm.21121.
Effect-site concentration of remimazolam at loss and recovery of responsiveness during general anesthesia: a simulation study
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 2Department of Anesthesiology and Pain Medicine, Daejeon Eulji Medical Center, Eulji University School of Medicine, Daejeon, Korea
- 3Clinical Pharmacology and Therapeutics, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2535318
- DOI: http://doi.org/10.17085/apm.21121
Abstract
- Background
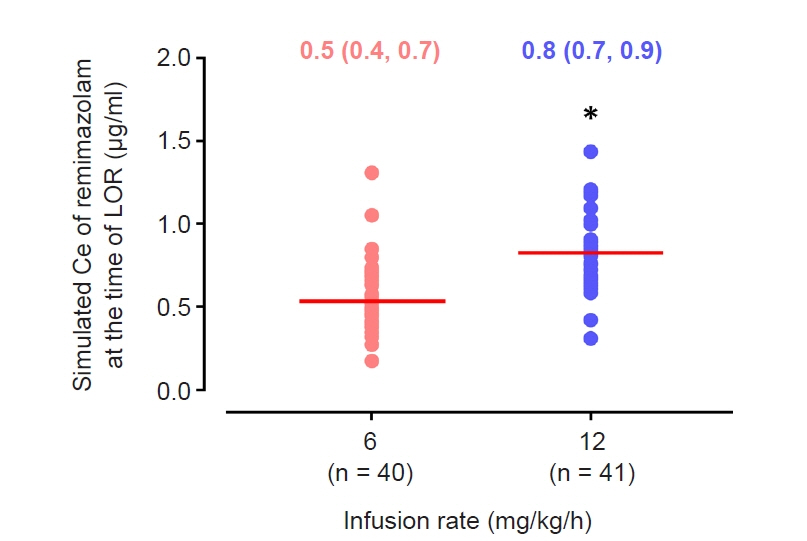
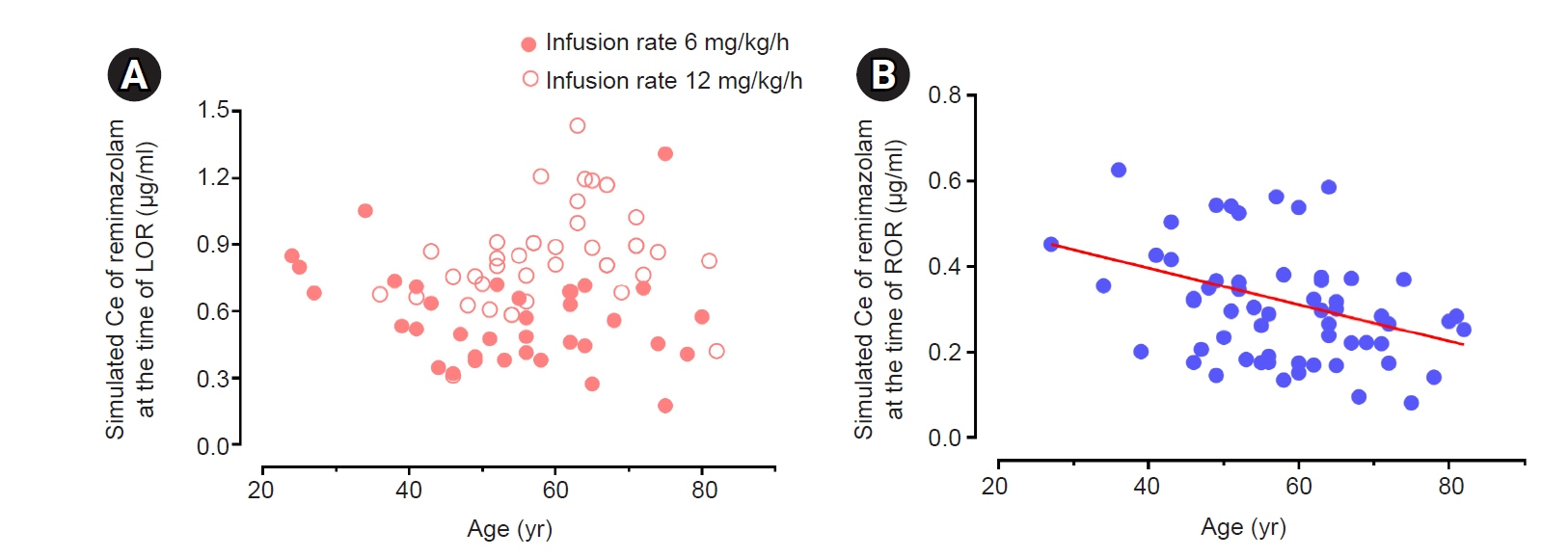
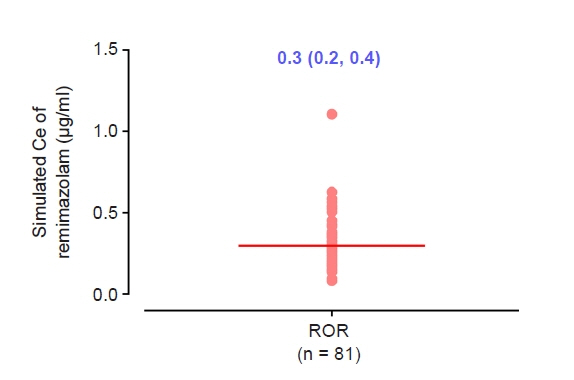
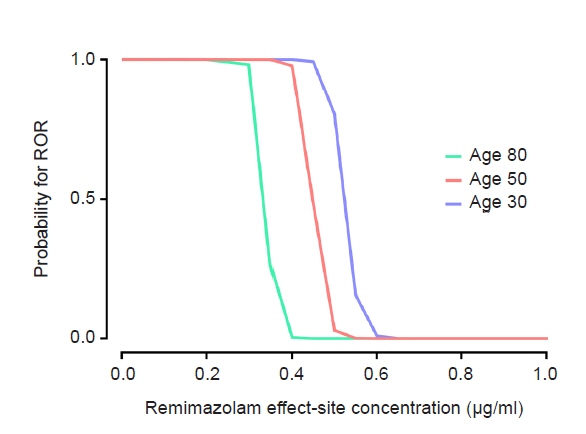
The objective of this study was to investigate the effect-site concentration (Ce) of remimazolam at loss of response (LOR) and recovery of response (ROR) in patients underwent general anesthesia using simulation. In addition, the relationships between patient’s factors and simulated Ce at LOR and ROR were examined. Methods: The medical records of 81 patients who underwent elective surgery under general anesthesia using remimazolam with simulation of Ce between August 4, 2021 and October 12, 2021, were retrospectively reviewed. Remimazolam was administered as an induction dose of 6 or 12 mg/kg/h until the patient became unresponsive, followed by 0.3–2 mg/kg/ h to maintain BIS values below 60. Simultaneously, simulations of manual infusion mode were performed using Asan Pump software and the Ce of remimazolam was simulated using the Schüttler model. Whenever infusion rate of remimazolam was manually changed, the simulated Ce was confirmed almost simultaneously. LOR and ROR, defined as unresponsive and eye-opening to verbal commands, respectively, were recorded in the Asan Pump program. Results: The median (1Q, 3Q) simulated Ce at LOR and ROR were 0.7 (0.5, 0.9) and 0.3 (0.2, 0.4) μg/ml, respectively. LOR was achieved in 1.9 min after remimazolam infusion with cumulative doses of 0.3 mg/kg. There was a significant relationship between age and simulated Ce at ROR (Ce at ROR = –0.0043 × age + 0.57, r = 0.30, P = 0.014). Conclusions: For optimal dosage adjustment, simulating Ce while administering remimazolam with a weight-based dose during anesthesia is helpful. Elderly patients may recover from anesthesia at lower Ce of remimazolam.
Keyword
Figure
Reference
-
1. Doi M, Hirata N, Suzuki T, Morisaki H, Morimatsu H, Sakamoto A. Safety and efficacy of remimazolam in induction and maintenance of general anesthesia in high-risk surgical patients (ASA Class III): results of a multicenter, randomized, double-blind, parallel-group comparative trial. J Anesth. 2020; 34:491–501.
Article2. Doi M, Morita K, Takeda J, Sakamoto A, Yamakage M, Suzuki T. Efficacy and safety of remimazolam versus propofol for general anesthesia: a multicenter, single-blind, randomized, parallel-group, phase IIb/III trial. J Anesth. 2020; 34:543–53.
Article3. Liu T, Lai T, Chen J, Lu Y, He F, Chen Y, et al. Effect of remimazolam induction on hemodynamics in patients undergoing valve replacement surgery: a randomized, double-blind, controlled trial. Pharmacol Res Perspect. 2021; 9:e00851.
Article4. Nakanishi T, Sento Y, Kamimura Y, Tsuji T, Kako E, Sobue K. Remimazolam for induction of anesthesia in elderly patients with severe aortic stenosis: a prospective, observational pilot study. BMC Anesthesiol. 2021; 21:306.
Article5. Nakayama J, Ogihara T, Yajima R, Innami Y, Ouchi T. Anesthetic management of super-elderly patients with remimazolam: a report of two cases. JA Clin Rep. 2021; 7:71.
Article6. Antonik LJ, Goldwater DR, Kilpatrick GJ, Tilbrook GS, Borkett KM. A placebo- and midazolam-controlled phase I single ascending-dose study evaluating the safety, pharmacokinetics, and pharmacodynamics of remimazolam (CNS 7056): part I. Safety, efficacy, and basic pharmacokinetics. Anesth Analg. 2012; 115:274–83.7. Eisenried A, Schüttler J, Lerch M, Ihmsen H, Jeleazcov C. Pharmacokinetics and pharmacodynamics of remimazolam (CNS 7056) after continuous infusion in healthy male volunteers: part II. Pharmacodynamics of electroencephalogram effects. Anesthesiology. 2020; 132:652–66.8. Schüttler J, Eisenried A, Lerch M, Fechner J, Jeleazcov C, Ihmsen H. Pharmacokinetics and pharmacodynamics of remimazolam (CNS 7056) after continuous infusion in healthy male volunteers: part I. Pharmacokinetics and clinical pharmacodynamics. Anesthesiology. 2020; 132:636–51.9. Louizos C, Yáñez JA, Forrest ML, Davies NM. Understanding the hysteresis loop conundrum in pharmacokinetic/pharmacodynamic relationships. J Pharm Pharm Sci. 2014; 17:34–91.
Article10. Iwakiri H, Nishihara N, Nagata O, Matsukawa T, Ozaki M, Sessler DI. Individual effect-site concentrations of propofol are similar at loss of consciousness and at awakening. Anesth Analg. 2005; 100:107–10.
Article11. Sukumar V, Radhakrishnan A, Keshavan VH. Effect site concentration of propofol at induction and recovery of anaesthesia - a correlative dose-response study. Indian J Anaesth. 2018; 62:263–8.
Article12. Absalom AR, Glen JI, Zwart GJ, Schnider TW, Struys MM. Target-controlled infusion: a mature technology. Anesth Analg. 2016; 122:70–8.13. Minto CF, Schnider TW, Egan TD, Youngs E, Lemmens HJ, Gambus PL, et al. Influence of age and gender on the pharmacokinetics and pharmacodynamics of remifentanil. I. Model development. Anesthesiology. 1997; 86:10–23.
Article14. Beal SL, Sheiner LB. NONMEM users guide. San Francisco (CA): University of California;1992.15. Park JH, Choi SM, Park JH, Lee KH, Yun HJ, Lee EK, et al. Population pharmacokinetic analysis of propofol in underweight patients under general anaesthesia. Br J Anaesth. 2018; 121:559–66.
Article16. Holford NH, Sheiner LB. Understanding the dose-effect relationship: clinical application of pharmacokinetic-pharmacodynamic models. Clin Pharmacokinet. 1981; 6:429–53.17. Obara S. Simulation of residual sedation effect of remimazolam: pharmacokinetic-pharmacodynamic simulation can be an additional standard anesthesia monitoring method. J Anesth. 2022; 36:167–70.
Article18. Platten HP, Schweizer E, Dilger K, Mikus G, Klotz U. Pharmacokinetics and the pharmacodynamic action of midazolam in young and elderly patients undergoing tooth extraction. Clin Pharmacol Ther. 1998; 63:552–60.
Article19. Bertz RJ, Kroboth PD, Kroboth FJ, Reynolds IJ, Salek F, Wright CE, et al. Alprazolam in young and elderly men: sensitivity and tolerance to psychomotor, sedative and memory effects. J Pharmacol Exp Ther. 1997; 281:1317–29.20. Zhou J, Leonowens C, Ivaturi VD, Lohmer LL, Curd L, Ossig J, et al. Population pharmacokinetic/pharmacodynamic modeling for remimazolam in the induction and maintenance of general anesthesia in healthy subjects and in surgical subjects. J Clin Anesth. 2020; 66:109899.
Article21. Lohmer LL, Schippers F, Petersen KU, Stoehr T, Schmith VD. Time-to-event modeling for remimazolam for the indication of induction and maintenance of general anesthesia. J Clin Pharmacol. 2020; 60:505–14.
Article22. Minto CF, Schnider TW, Gregg KM, Henthorn TK, Shafer SL. Using the time of maximum effect site concentration to combine pharmacokinetics and pharmacodynamics. Anesthesiology. 2003; 99:324–33.
Article23. Han DW. Pharmacokinetic and pharmacodynamic modeling in anesthetic field. Anesth Pain Med. 2014; 9:77–86.24. Sanders RD, Tononi G, Laureys S, Sleigh JW. Unresponsiveness ≠ unconsciousness. Anesthesiology. 2012; 116:946–59.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Psychogenic coma after general anesthesia with remimazolam and remifentanil -a case report-
- Comparison of remimazolam–remifentanil and propofol–remifentanil during laparoscopic cholecystectomy
- Effects of remimazolam combined with remifentanil on quality of recovery after ambulatory hysteroscopic surgery: a prospective, observational study
- Effective concentration of remifentanil for successful i-gel insertion during remimazolam induction
- Remimazolam – current knowledge on a new intravenous benzodiazepine anesthetic agent