Cardiovasc Prev Pharmacother.
2022 Oct;4(4):142-148. 10.36011/cpp.2022.4.e18.
The effects and side effects of liraglutide as a treatment for obesity
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 2Department of Medical Informatics, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2534487
- DOI: http://doi.org/10.36011/cpp.2022.4.e18
Abstract
- The incidence of obesity is increasing throughout the world, including Korea. Liraglutide, the main purpose of which is glucose control, has recently gained significant attention due to its additional effect on weight loss. Liraglutide injections have been widely used as an important treatment for obese patients in Korea. In addition to weight loss, liraglutide has various other effects, such as prevention of cardiovascular disease. Despite its excellent effect on weight loss, notable side effects, such as nausea and vomiting, have also been associated with liraglutide. Despite these side effects, liraglutide has not been discontinued due to its beneficial effects on weight loss. Nonetheless, there are reports wherein patients did not experience weight loss upon taking the drug. As such, there is a possibility of liraglutide misuse and abuse. Therefore, physicians need to have a broad understanding of liraglutide and understand the advantages and disadvantages of liraglutide prescription.
Figure
Reference
-
1. Kang HT, Shim JY, Lee HR, Park BJ, Linton JA, Lee YJ. Trends in prevalence of overweight and obesity in Korean adults, 1998-2009: the Korean National Health and Nutrition Examination Survey. J Epidemiol. 2014; 24:109–16.2. Finkelstein EA, Khavjou OA, Thompson H, Trogdon JG, Pan L, Sherry B, et al. Obesity and severe obesity forecasts through 2030. Am J Prev Med. 2012; 42:563–70.
Article3. Logue J, Murray HM, Welsh P, Shepherd J, Packard C, Macfarlane P, et al. Obesity is associated with fatal coronary heart disease independently of traditional risk factors and deprivation. Heart. 2011; 97:564–8.
Article4. Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006; 444:881–7.
Article5. Yang YS, Han BD, Han K, Jung JH, Son JW; Taskforce Team of the Obesity Fact Sheet of the Korean Society for the Study of Obesity. Obesity Fact Sheet in Korea, 2021: trends in obesity prevalence and obesity-related comorbidity incidence stratified by age from 2009 to 2019. J Obes Metab Syndr. 2022; 31:169–77.
Article6. Kim BY, Kang SM, Kang JH, Kang SY, Kim KK, Kim KB, et al. 2020 Korean Society for the Study of Obesity Guidelines for the Management of Obesity in Korea. J Obes Metab Syndr. 2021; 30:81–92.
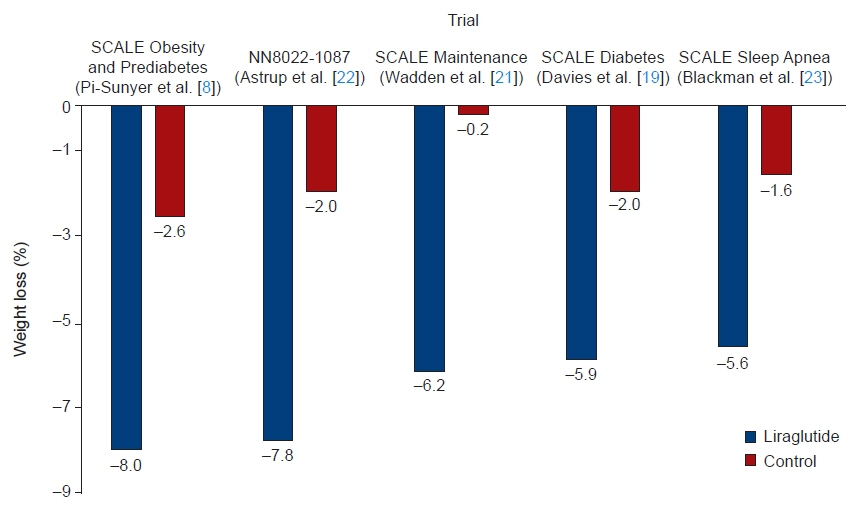
Article7. Astrup A, Rossner S, Van Gaal L, Rissanen A, Niskanen L, Al Hakim M, et al. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet. 2009; 374:1606–16.
Article8. Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf M, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015; 373:11–22.
Article9. Gorgojo-Martinez JJ, Basagoiti-Carreno B, Sanz-Velasco A, Serrano-Moreno C, Almodovar-Ruiz F. Effectiveness and tolerability of orlistat and liraglutide in patients with obesity in a real-world setting: the XENSOR Study. Int J Clin Pract. 2019; 73:e13399.
Article10. Mojsov S, Heinrich G, Wilson IB, Ravazzola M, Orci L, Habener JF. Preproglucagon gene expression in pancreas and intestine diversifies at the level of post-translational processing. J Biol Chem. 1986; 261:11880–9.
Article11. Kreymann B, Williams G, Ghatei MA, Bloom SR. Glucagon-like peptide-1 7-36: a physiological incretin in man. Lancet. 1987; 2:1300–4.
Article12. Meier JJ. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2012; 8:728–42.
Article13. Nauck MA, Heimesaat MM, Behle K, Holst JJ, Nauck MS, Ritzel R, et al. Effects of glucagon-like peptide 1 on counterregulatory hormone responses, cognitive functions, and insulin secretion during hyperinsulinemic, stepped hypoglycemic clamp experiments in healthy volunteers. J Clin Endocrinol Metab. 2002; 87:1239–46.
Article14. Simpson KA, Martin NM, Bloom SR. Hypothalamic regulation of appetite. Expert Rev Endocrinol Metab. 2008; 3:577–92.
Article15. Krieger JP. Intestinal glucagon-like peptide-1 effects on food intake: physiological relevance and emerging mechanisms. Peptides. 2020; 131:170342.
Article16. Hutch CR, Sandoval D. The role of GLP-1 in the metabolic success of bariatric surgery. Endocrinology. 2017; 158:4139–51.
Article17. Knudsen LB, Lau J. The discovery and development of liraglutide and semaglutide. Front Endocrinol (Lausanne). 2019; 10:155.
Article18. Patel D, Smith A. Patient initiation and maintenance of GLP-1 RAs for treatment of obesity. Expert Rev Clin Pharmacol. 2021; 14:1193–204.
Article19. Davies MJ, Bergenstal R, Bode B, Kushner RF, Lewin A, Skjoth TV, et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE Diabetes randomized clinical trial. JAMA. 2015; 314:687–99.
Article20. le Roux CW, Astrup A, Fujioka K, Greenway F, Lau DC, Van Gaal L, et al. 3 Years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: a randomised, double-blind trial. Lancet. 2017; 389:1399–409.21. Wadden TA, Hollander P, Klein S, Niswender K, Woo V, Hale PM, et al. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the SCALE Maintenance randomized study. Int J Obes (Lond). 2013; 37:1443–51.
Article22. Astrup A, Carraro R, Finer N, Harper A, Kunesova M, Lean ME, et al. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int J Obes (Lond). 2012; 36:843–54.
Article23. Blackman A, Foster GD, Zammit G, Rosenberg R, Aronne L, Wadden T, et al. Effect of liraglutide 3.0 mg in individuals with obesity and moderate or severe obstructive sleep apnea: the SCALE Sleep Apnea randomized clinical trial. Int J Obes (Lond). 2016; 40:1310–9.
Article24. Wadden TA, Tronieri JS, Sugimoto D, Lund MT, Auerbach P, Jensen C, et al. Liraglutide 3.0 mg and intensive behavioral therapy (IBT) for obesity in primary care: the SCALE IBT randomized controlled trial. Obesity (Silver Spring). 2020; 28:529–36.
Article25. Garvey WT, Birkenfeld AL, Dicker D, Mingrone G, Pedersen SD, Satylganova A, et al. Efficacy and safety of liraglutide 3.0 mg in individuals with overweight or obesity and type 2 diabetes treated with basal insulin: the SCALE Insulin randomized controlled trial. Diabetes Care. 2020; 43:1085–93.
Article26. Park JS, Kwon J, Choi HJ, Lee C. Clinical effectiveness of liraglutide on weight loss in South Koreans: first real-world retrospective data on Saxenda in Asia. Medicine (Baltimore). 2021; 100:e23780.27. Rondanelli M, Perna S, Astrone P, Grugnetti A, Solerte SB, Guido D. Twenty-four-week effects of liraglutide on body composition, adherence to appetite, and lipid profile in overweight and obese patients with type 2 diabetes mellitus. Patient Prefer Adherence. 2016; 10:407–13.28. Ge JJ, Wang DJ, Song W, Shen SM, Ge WH. The effectiveness and safety of liraglutide in treating overweight/obese patients with polycystic ovary syndrome: a meta-analysis. J Endocrinol Invest. 2022; 45:261–73.29. Davies MJ, Aronne LJ, Caterson ID, Thomsen AB, Jacobsen PB, Marso SP, et al. Liraglutide and cardiovascular outcomes in adults with overweight or obesity: a post hoc analysis from SCALE randomized controlled trials. Diabetes Obes Metab. 2018; 20:734–9.30. Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016; 375:311–22.31. Novo Nordisk. Victoza (liraglutide) prescribing information [Internet]. Plainsboro: Novo Nordisk;2022. [cited 2022 Aug 12]. Available from: https://www.novo-pi.com/victoza.pdf.32. Patel DK, Stanford FC. Safety and tolerability of new-generation anti- obesity medications: a narrative review. Postgrad Med. 2018; 130:173–82.33. Min J, Shinn J, Kim HS. Development of a predictive model for the side effects of liraglutide. Cardiovasc Prev Pharmacother. 2022; 4:87–93.34. Everhart JE. Contributions of obesity and weight loss to gallstone disease. Ann Intern Med. 1993; 119:1029–35.
Article35. Nauck MA, Jensen TJ, Rosenkilde C, Calanna S, Buse JB; LEADER Publication Committee on behalf of the LEADER Trial Investigators. Neoplasms reported with liraglutide or placebo in people with type 2 diabetes: results from the LEADER randomized trial. Diabetes Care. 2018; 41:1663–71.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Side Effects Associated with Liraglutide Treatment for Obesity as Well as Diabetes
- Development of a predictive model for the side effects of liraglutide
- Recent Advances in Anti-Obesity Agents
- Safety of Anti-Obesity Drugs Approved for Long-Term Use
- Effectiveness and Safety of Liraglutide Treatment in Patients with a Psychiatric Disorder