J Liver Cancer.
2022 Sep;22(2):167-177. 10.17998/jlc.2022.09.19.
The diagnostic value of circulating tumor DNA in hepatitis B virus induced hepatocellular carcinoma: a systematic review and meta-analysis
- Affiliations
-
- 1Institute for Digestive Research, Digestive Disease Center, Department of Internal Medicine, Soonchunhyang University College of Medicine, Seoul, Korea
- 2Department of Internal Medicine, College of Medicine, Chungnam National University, Daejeon, Korea
- 3Department of Internal Medicine, Guro Hospital, Korea University College of Medicine, Seoul, Korea
- 4Department of Internal Medicine, St. Vincent’s Hospital, College of Medicine, The Catholic University of Korea, Suwon, Korea
- 5Department of Internal Medicine and Liver Research Institute, Seoul National University College of Medicine, Seoul, Korea
- 6Department of Internal Medicine, Soonchunhyang University College of Medicine, Cheonan, Korea
- 7Department of Internal Medicine, Seoul Metropolitan Government Seoul National University Boramae Medical Center, Seoul National University College of Medicine, Seoul, Korea
- 8Department of Internal Medicine, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- 9Department of Internal Medicine, Soonchunhyang University College of Medicine, Bucheon, Korea
- 10Soonchunhyang Institute of Medi-bio Science (SIMS), Soonchunhyang University, Cheonan, Korea
- 11Department of Biostatistics, Soonchunhyang University Hospital, Seoul, Korea
- 12Department of Applied Statistics, Chung-Ang University, Seoul, Korea
- KMID: 2534243
- DOI: http://doi.org/10.17998/jlc.2022.09.19
Abstract
- Background
/Aim: New biomarkers are urgently needed to aid in the diagnosis of early stage hepatocellular carcinoma (HCC). We performed a meta-analysis on the diagnostic utility of circulating tumor DNA (ctDNA) levels in patients with hepatitis B virus-induced HCC.
Methods
We retrieved relevant articles from PubMed, Embase, and the Cochrane Library up to February 8, 2022. Two subgroups were defined; one subset of studies analyzed the ctDNA methylation status, and the other subset combined tumor markers and ctDNA assays. Pooled sensitivity (SEN), specificity (SPE), positive likelihood ratio (PLR), negative likelihood ratio (NLR), diagnostic odds ratio (DOR), and area under the summary receiver operating characteristic curve (AUC) were analyzed.
Results
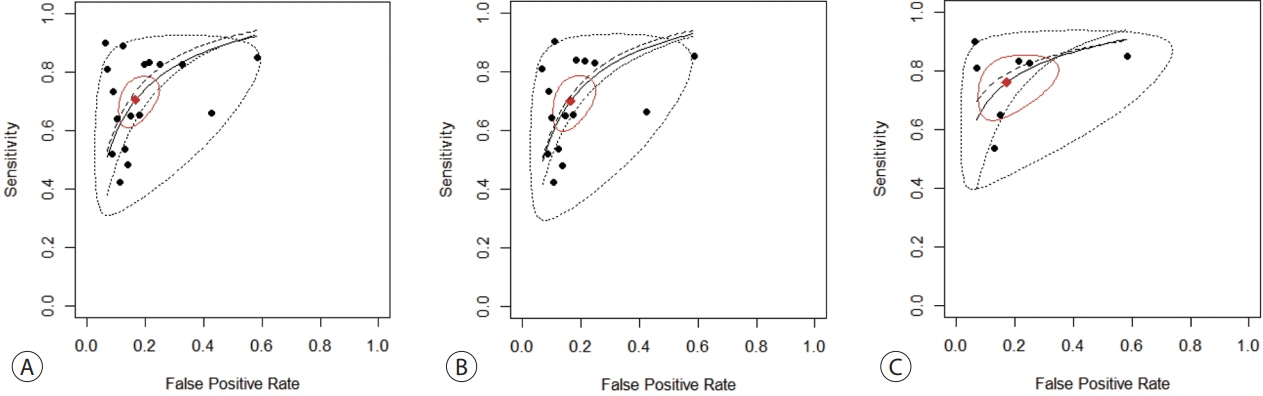
Nine articles including 2,161 participants were included. The overall SEN and SPE were 0.705 (95% confidence interval [CI], 0.629-0.771) and 0.833 (95% CI, 0.769-0.882), respectively. The DOR, PLR, and NLR were 11.759 (95% CI, 7.982-17.322), 4.285 (95% CI, 3.098- 5.925), and 0.336 (0.301-0.366), respectively. The ctDNA assay subset exhibited an AUC of 0.835. The AUC of the combined tumor marker and ctDNA assay was 0.848, with an SEN of 0.761 (95% CI, 0.659-0.839) and an SPE of 0.828 (95% CI, 0.692-0.911).
Conclusions
Circulating tumor DNA has promising diagnostic potential for HCC. It can serve as an auxiliary tool for HCC screening and detection, especially when combined with tumor markers.
Figure
Reference
-
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021; 71:209–249.2. Center MM, Jemal A. International trends in liver cancer incidence rates. Cancer Epidemiol Biomarkers Prev. 2011; 20:2362–2368.3. Ghouri YA, Mian I, Rowe JH. Review of hepatocellular carcinoma: epidemiology, etiology, and carcinogenesis. J Carcinog. 2017; 16:1.4. Korean Liver Cancer Association; National Cancer Center. 2018 Korean Liver Cancer Association-National Cancer Center Korea practice guidelines for the management of hepatocellular carcinoma. Gut Liver. 2019; 13:227–299.5. European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018; 69:182–236.6. Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018; 67:358–380.7. Chou R, Cuevas C, Fu R, Devine B, Wasson N, Ginsburg A, et al. Imaging techniques for the diagnosis of hepatocellular carcinoma: a systematic review and meta-analysis. Ann Intern Med. 2015; 162:697–711.8. Singal AG, Nehra M, Adams-Huet B, Yopp AC, Tiro JA, Marrero JA, et al. Detection of hepatocellular carcinoma at advanced stages among patients in the HALT-C trial: where did surveillance fail? Am J Gastroenterol. 2013; 108:425–432.9. Colli A, Fraquelli M, Casazza G, Massironi S, Colucci A, Conte D, et al. Accuracy of ultrasonography, spiral CT, magnetic resonance, and alpha-fetoprotein in diagnosing hepatocellular carcinoma: a systematic review. Am J Gastroenterol. 2006; 101:513–523.10. Mezzalira S, De Mattia E, Guardascione M, Dalle Fratte C, Cecchin E, Toffoli G. Circulating-free DNA analysis in hepatocellular carcinoma: a promising strategy to improve patients’ management and therapy outcomes. Int J Mol Sci. 2019; 20:5498.11. Li X, Wang H, Li T, Wang L, Wu X, Liu J, et al. Circulating tumor DNA/circulating tumor cells and the applicability in different causes induced hepatocellular carcinoma. Curr Probl Cancer. 2020; 44:100516.12. Alunni-Fabbroni M, Rönsch K, Huber T, Cyran CC, Seidensticker M, Mayerle J, et al. Circulating DNA as prognostic biomarker in patients with advanced hepatocellular carcinoma: a translational exploratory study from the SORAMIC trial. J Transl Med. 2019; 17:328.13. Mody K, Kasi PM, Yang JD, Surapaneni PK, Ritter A, Roberts A, et al. Feasibility of circulating tumor DNA testing in hepatocellular carcinoma. J Gastrointest Oncol. 2019; 10:745–750.14. Chan KC, Jiang P, Zheng YW, Liao GJ, Sun H, Wong J, et al. Cancer genome scanning in plasma: detection of tumor-associated copy number aberrations, single-nucleotide variants, and tumoral heterogeneity by massively parallel sequencing. Clin Chem. 2013; 59:211–224.15. Labgaa I, Villacorta-Martin C, D’Avola D, Craig AJ, von Felden J, Martins-Filho SN, et al. A pilot study of ultra-deep targeted sequencing of plasma DNA identifies driver mutations in hepatocellular carcinoma. Oncogene. 2018; 37:3740–3752.16. Ng CKY, Di Costanzo GG, Tosti N, Paradiso V, Coto-Llerena M, Roscigno G, et al. Genetic profiling using plasma-derived cell-free DNA in therapy-naïve hepatocellular carcinoma patients: a pilot study. Ann Oncol. 2018; 29:1286–1291.17. Heitzer E, Haque IS, Roberts CES, Speicher MR. Current and future perspectives of liquid biopsies in genomics-driven oncology. Nat Rev Genet. 2019; 20:71–88.18. Cohen JD, Douville C, Dudley JC, Mog BJ, Popoli M, Ptak J, et al. Detection of low-frequency DNA variants by targeted sequencing of the Watson and Crick strands. Nat Biotechnol. 2021; 39:1220–1227.19. Qu C, Wang Y, Wang P, Chen K, Wang M, Zeng H, et al. Detection of early-stage hepatocellular carcinoma in asymptomatic HBsAgseropositive individuals by liquid biopsy. Proc Natl Acad Sci U S A. 2019; 116:6308–6312.20. Cohen JD, Li L, Wang Y, Thoburn C, Afsari B, Danilova L, et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science. 2018; 359:926–930.21. Liu MC, Oxnard GR, Klein EA, Swanton C, Seiden MV; CCGA Consortium. Sensitive and specific multi-cancer detection and localization using methylation signatures in cell-free DNA. Ann Oncol. 2020; 31:745–759.22. Mah WC, Lee CG. DNA methylation: potential biomarker in hepatocellular carcinoma. Biomark Res. 2014; 2:5.23. Adeniji N, Dhanasekaran R. Current and emerging tools for hepatocellular carcinoma surveillance. Hepatol Commun. 2021; 5:1972–1986.24. Branco MR, Ficz G, Reik W. Uncovering the role of 5-hydroxymethylcytosine in the epigenome. Nat Rev Genet. 2011; 13:7–13.25. Zeng C, Stroup EK, Zhang Z, Chiu BC, Zhang W. Towards precision medicine: advances in 5-hydroxymethylcytosine cancer biomarker discovery in liquid biopsy. Cancer Commun (Lond). 2019; 39:12.26. Gao P, Lin S, Cai M, Zhu Y, Song Y, Sui Y, et al. 5-Hydroxymethylcytosine profiling from genomic and cell-free DNA for colorectal cancers patients. J Cell Mol Med. 2019; 23:3530–3537.27. Li W, Zhang X, Lu X, You L, Song Y, Luo Z, et al. 5-Hydroxymethylcytosine signatures in circulating cell-free DNA as diagnostic biomarkers for human cancers. Cell Res. 2017; 27:1243–1257.28. Song CX, Yin S, Ma L, Wheeler A, Chen Y, Zhang Y, et al. 5-Hydroxymethylcytosine signatures in cell-free DNA provide information about tumor types and stages. Cell Res. 2017; 27:1231–1242.29. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009; 6:e1000097.30. Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011; 155:529–536.31. Cai J, Chen L, Zhang Z, Zhang X, Lu X, Liu W, et al. Genome-wide mapping of 5-hydroxymethylcytosines in circulating cell-free DNA as a non-invasive approach for early detection of hepatocellular carcinoma. Gut. 2019; 68:2195–2205.32. Dong X, Hou Q, Chen Y, Wang X. Diagnostic value of the methylation of multiple gene promoters in serum in hepatitis B virus-related hepatocellular carcinoma. Dis Markers. 2017; 2017:2929381.33. Hu N, Fan XP, Fan YC, Chen LY, Qiao CY, Han LY, et al. Hypomethylated ubiquitin-conjugating enzyme2 Q1 (UBE2Q1) gene promoter in the serum is a promising biomarker for hepatitis B virus-associated hepatocellular carcinoma. Tohoku J Exp Med. 2017; 242:93–100.34. Dong X, He H, Zhang W, Yu D, Wang X, Chen Y. Combination of serum RASSF1A methylation and AFP is a promising non-invasive biomarker for HCC patient with chronic HBV infection. Diagn Pathol. 2015; 10:133.35. Li F, Fan YC, Gao S, Sun FK, Yang Y, Wang K. Methylation of serum insulin-like growth factor-binding protein 7 promoter in hepatitis B virus-associated hepatocellular carcinoma. Genes Chromosomes Cancer. 2014; 53:90–97.36. Han LY, Fan YC, Mu NN, Gao S, Li F, Ji XF, et al. Aberrant DNA methylation of G-protein-coupled bile acid receptor Gpbar1 (TGR5) is a potential biomarker for hepatitis B Virus associated hepatocellular carcinoma. Int J Med Sci. 2014; 11:164–171.37. Yang Y, Fan YC, Gao S, Dou CY, Zhang JJ, Sun FK, et al. Methylated cysteine dioxygenase-1 gene promoter in the serum is a potential biomarker for hepatitis B virus-related hepatocellular carcinoma. Tohoku J Exp Med. 2014; 232:187–194.38. Zhang P, Wen X, Gu F, Deng X, Li J, Dong J, et al. Methylation profiling of serum DNA from hepatocellular carcinoma patients using an Infinium Human Methylation 450 BeadChip. Hepatol Int. 2013; 7:893–900.39. Mann J, Reeves HL, Feldstein AE. Liquid biopsy for liver diseases. Gut. 2018; 67:2204–2212.40. Ye Q, Ling S, Zheng S, Xu X. Liquid biopsy in hepatocellular carcinoma: circulating tumor cells and circulating tumor DNA. Mol Cancer. 2019; 18:114.41. Farinati F, Marino D, De Giorgio M, Baldan A, Cantarini M, Cursaro C, et al. Diagnostic and prognostic role of alpha-fetoprotein in hepatocellular carcinoma: both or neither? Am J Gastroenterol. 2006; 101:524–532.42. Zhang Z, Chen P, Xie H, Cao P. Using circulating tumor DNA as a novel biomarker to screen and diagnose hepatocellular carcinoma: a systematic review and meta-analysis. Cancer Med. 2020; 9:1349–1364.43. Glas AS, Lijmer JG, Prins MH, Bonsel GJ, Bossuyt PM. The diagnostic odds ratio: a single indicator of test performance. J Clin Epidemiol. 2003; 56:1129–1135.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The use of transient elastography for predicting hepatocellular carcinoma in chronic hepatitis B patients: Editorial on “Risk assessment of hepatitis B virus-related hepatocellular carcinoma development using vibration-controlled transient elastography: Systematic review and meta-analysis”
- Hepatocellular carcinoma surveillance after sustained virological response in chronic hepatitis C: Editorial on “Non-invasive prediction of post-sustained virological response hepatocellular carcinoma in hepatitis C virus: A systematic review and meta-analysis”
- Metabolic dysfunction-associated fatty liver disease is a ubiquitous latent cofactor in viral- and alcoholic-related hepatocellular carcinoma: Editorial on “Global prevalence of metabolic dysfunction-associated fatty liver disease-related hepatocellular carcinoma: A systematic review and meta-analysis”
- A case of primary hepatocellular carcinoma following vertical transmission of hepatitis B virus in a child
- Reply to correspondence on “Risk assessment of hepatitis B virus-related hepatocellular carcinoma development using vibration-controlled transient elastography: Systematic review and meta-analysis”