Cancer Res Treat.
2022 Oct;54(4):1230-1239. 10.4143/crt.2021.1011.
Outcome of Intensive Therapy for Children with Relapsed Acute Myeloid Leukemia: A Single Institution Korean Study
- Affiliations
-
- 1Division of Hematology and Oncology, Department of Pediatrics, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2534202
- DOI: http://doi.org/10.4143/crt.2021.1011
Abstract
- Purpose
Approximately 30%-40% of pediatric acute myeloid leukemia (AML) patients relapse. In this study, we analyzed the outcome and prognostic factors of relapsed AML patients who had previously received first-line therapy at our institution.
Materials and Methods
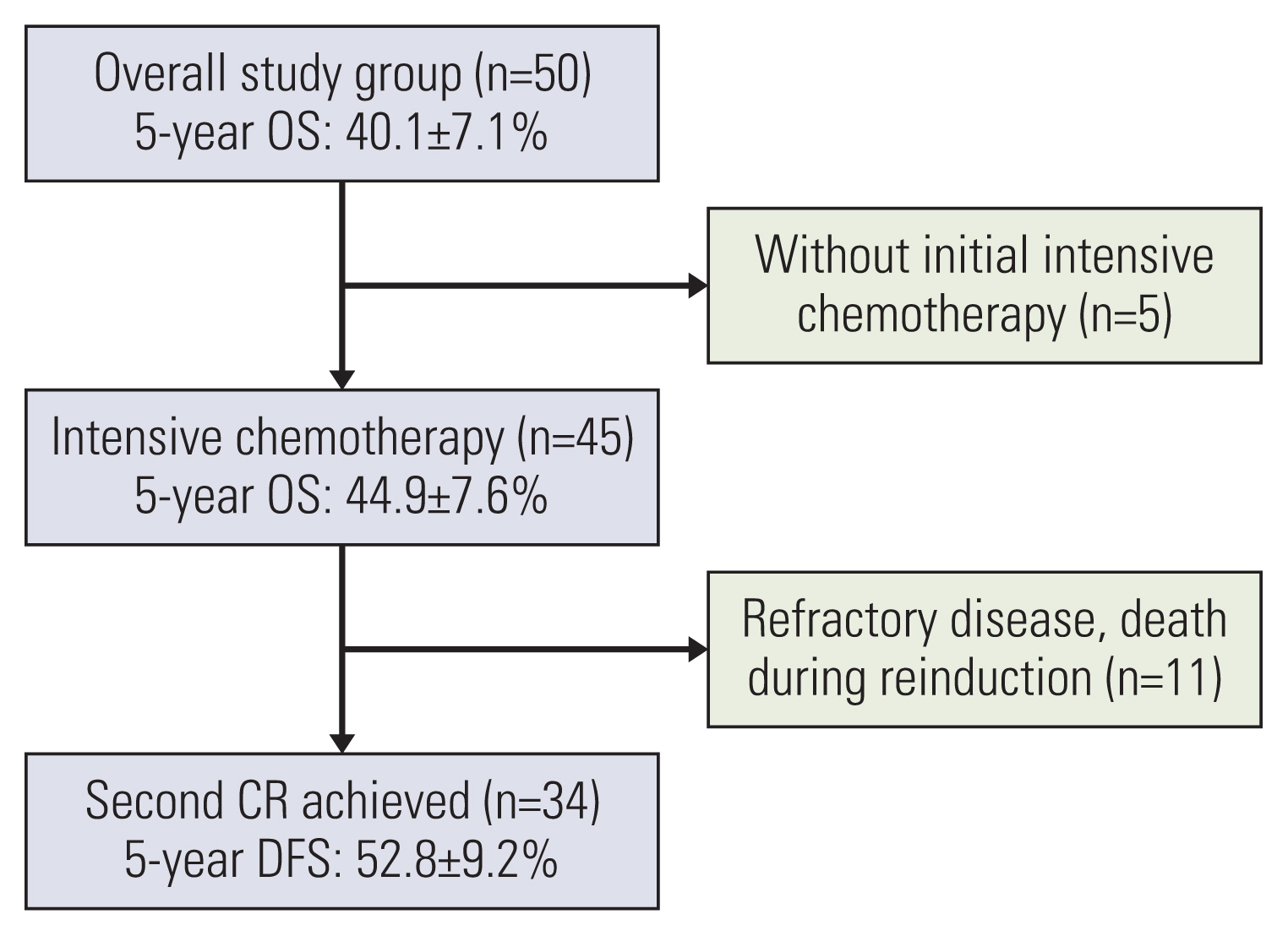
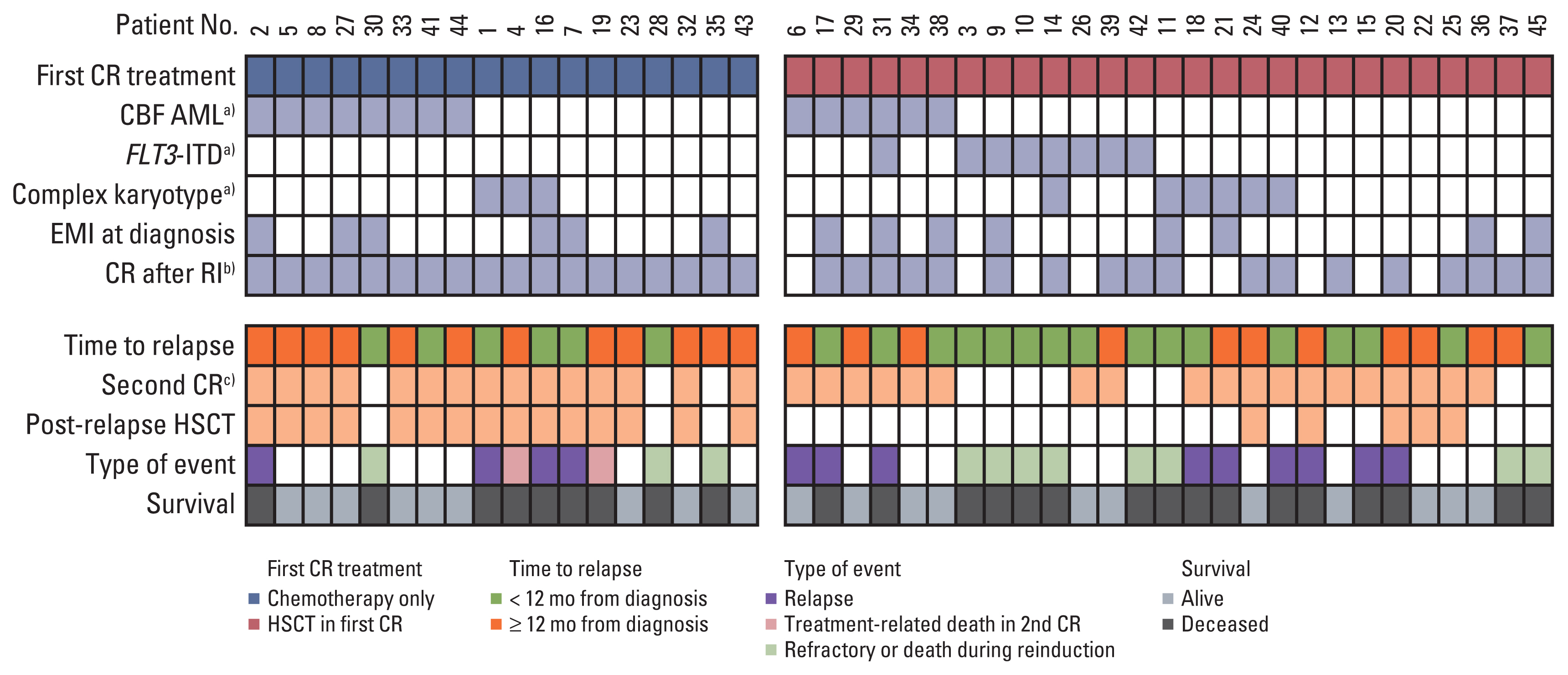
The study group consisted of 50 patients who had been diagnosed with AML from April 2009 to December 2018, and then showed first relapse. Thirty-two of the patients (64%) had previously received allogeneic hematopoietic stem cell transplantation (HSCT) in first complete remission (CR).
Results
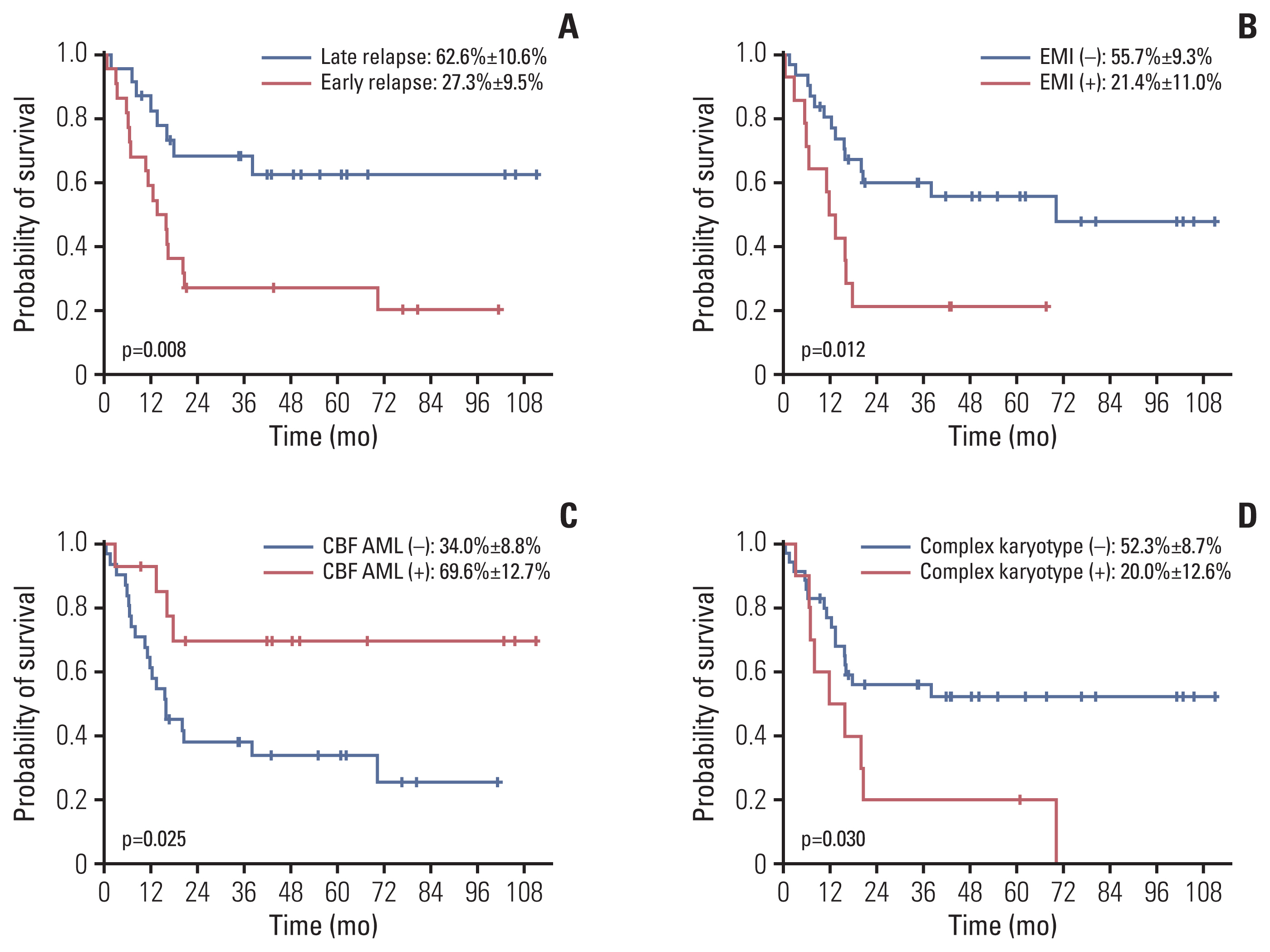
Forty-five of the patients (90%) received intensive chemotherapy upon diagnosis of relapse, and 76% (34/45) of these patients achieved a second CR. Estimated 5-year overall survival for these 45 patients was 44.9%±7.6%. Time from diagnosis to relapse, extramedullary involvement (EMI) at diagnosis, core binding factor AML, and complex karyotype were significant prognostic factors; in multivariate study, both time from diagnosis to relapse and EMI at diagnosis proved significant. There was no difference in 5-year disease-free survival between patients previously treated with chemotherapy only and those who received HSCT in first CR (52.4%±14.9% vs. 52.6%±11.5%). Of the 19 patients who achieved second CR after previous allogeneic HSCT in first CR and subsequent relapse, 11 were treated with chemotherapy only, and seven survive disease-free.
Conclusion
Intensive therapy allowed for long-term survival in 40%-50% of patients, and 50% of patients who achieved second CR, regardless of prior treatment modalities in first CR. Intensive treatment may allow for salvage of a significant portion of patients with relapsed pediatric AML.
Keyword
Figure
Reference
-
References
1. Rasche M, Zimmermann M, Borschel L, Bourquin JP, Dworzak M, Klingebiel T, et al. Successes and challenges in the treatment of pediatric acute myeloid leukemia: a retrospective analysis of the AML-BFM trials from 1987 to 2012. Leukemia. 2018; 32:2167–77.
Article2. Webb DK, Wheatley K, Harrison G, Stevens RF, Hann IM. Outcome for children with relapsed acute myeloid leukaemia following initial therapy in the Medical Research Council (MRC) AML 10 trial. MRC Childhood Leukaemia Working Party. Leukemia. 1999; 13:25–31.
Article3. Rubnitz JE, Razzouk BI, Lensing S, Pounds S, Pui CH, Ribeiro RC. Prognostic factors and outcome of recurrence in childhood acute myeloid leukemia. Cancer. 2007; 109:157–63.
Article4. Sander A, Zimmermann M, Dworzak M, Fleischhack G, von Neuhoff C, Reinhardt D, et al. Consequent and intensified relapse therapy improved survival in pediatric AML: results of relapse treatment in 379 patients of three consecutive AML-BFM trials. Leukemia. 2010; 24:1422–8.
Article5. Karlsson L, Forestier E, Hasle H, Jahnukainen K, Jonsson OG, Lausen B, et al. Outcome after intensive reinduction therapy and allogeneic stem cell transplant in paediatric relapsed acute myeloid leukaemia. Br J Haematol. 2017; 178:592–602.
Article6. Moritake H, Tanaka S, Miyamura T, Nakayama H, Shiba N, Shimada A, et al. The outcomes of relapsed acute myeloid leukemia in children: results from the Japanese Pediatric Leukemia/Lymphoma Study Group AML-05R study. Pediatr Blood Cancer. 2021; 68:e28736.
Article7. Rasche M, Zimmermann M, Steidel E, Alonzo T, Aplenc R, Bourquin JP, et al. Survival following relapse in children with acute myeloid leukemia: a report from AML-BFM and COG. Cancers (Basel). 2021; 13:2336.
Article8. Kaspers GJ, Zimmermann M, Reinhardt D, Gibson BE, Tamminga RY, Aleinikova O, et al. Improved outcome in pediatric relapsed acute myeloid leukemia: results of a randomized trial on liposomal daunorubicin by the International BFM Study Group. J Clin Oncol. 2013; 31:599–607.
Article9. Creutzig U, Zimmermann M, Dworzak MN, Gibson B, Tamminga R, Abrahamsson J, et al. The prognostic significance of early treatment response in pediatric relapsed acute myeloid leukemia: results of the international study Relapsed AML 2001/01. Haematologica. 2014; 99:1472–8.
Article10. Lee JW, Jang PS, Chung NG, Cho B, Kim HK. Treatment of children with acute myeloid leukaemia who relapsed after allogeneic haematopoietic stem cell transplantation. Br J Haematol. 2013; 160:80–6.
Article11. Lee JW, Kim S, Jang PS, Chung NG, Cho B, Im SA, et al. Prognostic role of postinduction minimal residual disease and myeloid sarcoma type extramedullary involvement in pediatric RUNX1-RUNX1T1 (+) acute myeloid leukemia. J Pediatr Hematol Oncol. 2020; 42:e132–9.
Article12. Ravandi F, Cortes JE, Jones D, Faderl S, Garcia-Manero G, Konopleva MY, et al. Phase I/II study of combination therapy with sorafenib, idarubicin, and cytarabine in younger patients with acute myeloid leukemia. J Clin Oncol. 2010; 28:1856–62.
Article13. Gorman MF, Ji L, Ko RH, Barnette P, Bostrom B, Hutchinson R, et al. Outcome for children treated for relapsed or refractory acute myelogenous leukemia (rAML): a Therapeutic Advances in Childhood Leukemia (TACL) Consortium study. Pediatr Blood Cancer. 2010; 55:421–9.
Article14. Fleischhack G, Hasan C, Graf N, Mann G, Bode U. IDA-FLAG (idarubicin, fludarabine, cytarabine, G-CSF), an effective remission-induction therapy for poor-prognosis AML of childhood prior to allogeneic or autologous bone marrow transplantation: experiences of a phase II trial. Br J Haematol. 1998; 102:647–55.
Article15. Niktoreh N, Lerius B, Zimmermann M, Gruhn B, Escherich G, Bourquin JP, et al. Gemtuzumab ozogamicin in children with relapsed or refractory acute myeloid leukemia: a report by Berlin-Frankfurt-Munster study group. Haematologica. 2019; 104:120–7.
Article16. Karol SE, Alexander TB, Budhraja A, Pounds SB, Canavera K, Wang L, et al. Venetoclax in combination with cytarabine with or without idarubicin in children with relapsed or refractory acute myeloid leukaemia: a phase 1, dose-escalation study. Lancet Oncol. 2020; 21:551–60.
Article17. Stove HK, Sandahl JD, Abrahamsson J, Asdahl PH, Forestier E, Ha SY, et al. Extramedullary leukemia in children with acute myeloid leukemia: a population-based cohort study from the Nordic Society of Pediatric Hematology and Oncology (NOPHO). Pediatr Blood Cancer. 2017; 64:e26520.
Article18. Sakaguchi H, Miyamura T, Tomizawa D, Taga T, Ishida H, Okamoto Y, et al. Effect of extramedullary disease on allogeneic hematopoietic cell transplantation for pediatric acute myeloid leukemia: a nationwide retrospective study. Bone Marrow Transplant. 2021; 56:1859–65.
Article19. Nakayama H, Tabuchi K, Tawa A, Tsukimoto I, Tsuchida M, Morimoto A, et al. Outcome of children with relapsed acute myeloid leukemia following initial therapy under the AML99 protocol. Int J Hematol. 2014; 100:171–9.
Article20. von Neuhoff C, Reinhardt D, Sander A, Zimmermann M, Bradtke J, Betts DR, et al. Prognostic impact of specific chromosomal aberrations in a large group of pediatric patients with acute myeloid leukemia treated uniformly according to trial AML-BFM 98. J Clin Oncol. 2010; 28:2682–9.
Article21. Selim A, Alvaro F, Cole CH, Fraser CJ, Mechinaud F, O’Brien TA, et al. Hematopoietic stem cell transplantation for children with acute myeloid leukemia in second remission: a report from the Australasian Bone Marrow Transplant Recipient Registry and the Australian and New Zealand Children’s Haematology Oncology Group. Pediatr Blood Cancer. 2019; 66:e27812.
Article22. Uden T, Bertaina A, Abrahamsson J, Ansari M, Balduzzi A, Bourquin JP, et al. Outcome of children relapsing after first allogeneic haematopoietic stem cell transplantation for acute myeloid leukaemia: a retrospective I-BFM analysis of 333 children. Br J Haematol. 2020; 189:745–50.
Article23. Yahng SA, Kim JH, Jeon YW, Yoon JH, Shin SH, Lee SE, et al. A well-tolerated regimen of 800 cGy TBI-fludarabine-busulfan-ATG for reliable engraftment after unmanipulated haploidentical peripheral blood stem cell transplantation in adult patients with acute myeloid leukemia. Biol Blood Marrow Transplant. 2015; 21:119–29.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Current treatment for pediatric acute myeloid leukemia
- A Case of Temporal Bone Myeloid Sarcoma
- Acute myeloid leukemia arising from chronic myelomonocytic leukemia during hypomethylating therapy
- Acute Myeloid Leukemia with Intracardiac Thrombus Presenting as Acute Limb Ischemia
- Remission induction therapy with TAD for acute myeloid leukemia