Cancer Res Treat.
2022 Oct;54(4):1148-1156. 10.4143/crt.2021.885.
Role of Esophagectomy after Chemoradiation Therapy in Patients with Locally Advanced Squamous Cell Carcinoma: A Comparative Analysis Stratified by Clinical Response to Chemoradiation Therapy
- Affiliations
-
- 1Department of Radiation Oncology, Kosin University Gospel Hospital, Kosin University College of Medicine, Busan, Korea
- 2Department of Radiation Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 3Department of Medical Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 4Department of Thoracic and Cardiovascular Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 5Department of Radiology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 6Division of Gastroenterology, Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2534194
- DOI: http://doi.org/10.4143/crt.2021.885
Abstract
- Purpose
This study aimed to evaluate the long-term effect of esophagectomy in patients with esophageal squamous cell carcinoma (ESCC) by comparing the chemoradiotherapy (CRT)-only group and the trimodality treatment (TMT) group who received concurrent CRT followed by surgery.
Materials and Methods
We included 412 operable ESCC patients treated with TMT or CRT between January 2005 and December 2015. The oncological outcomes of the two groups were compared using a weighted Cox proportional-hazards model with inverse probability of treatment weighting (IPTW).
Results
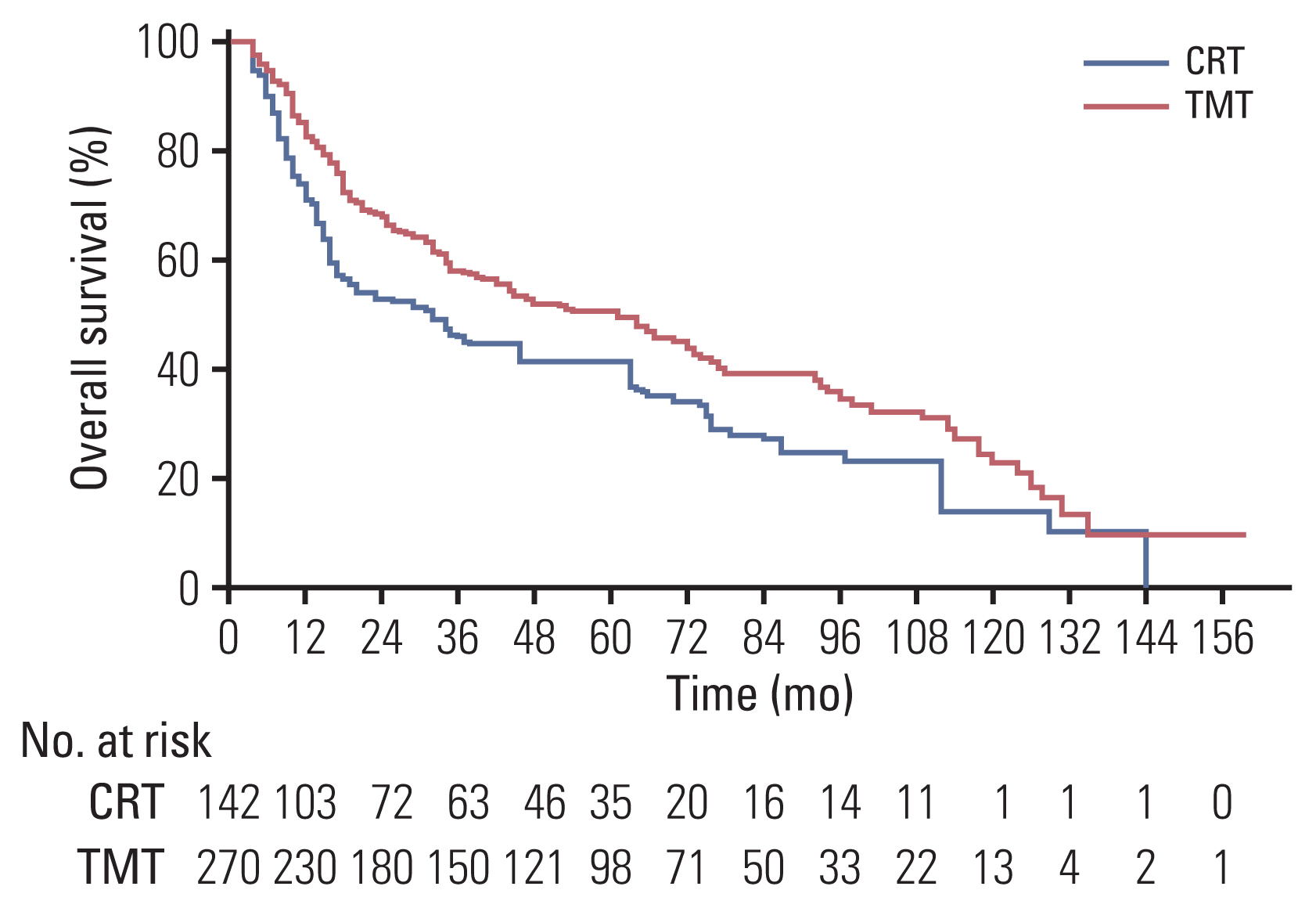
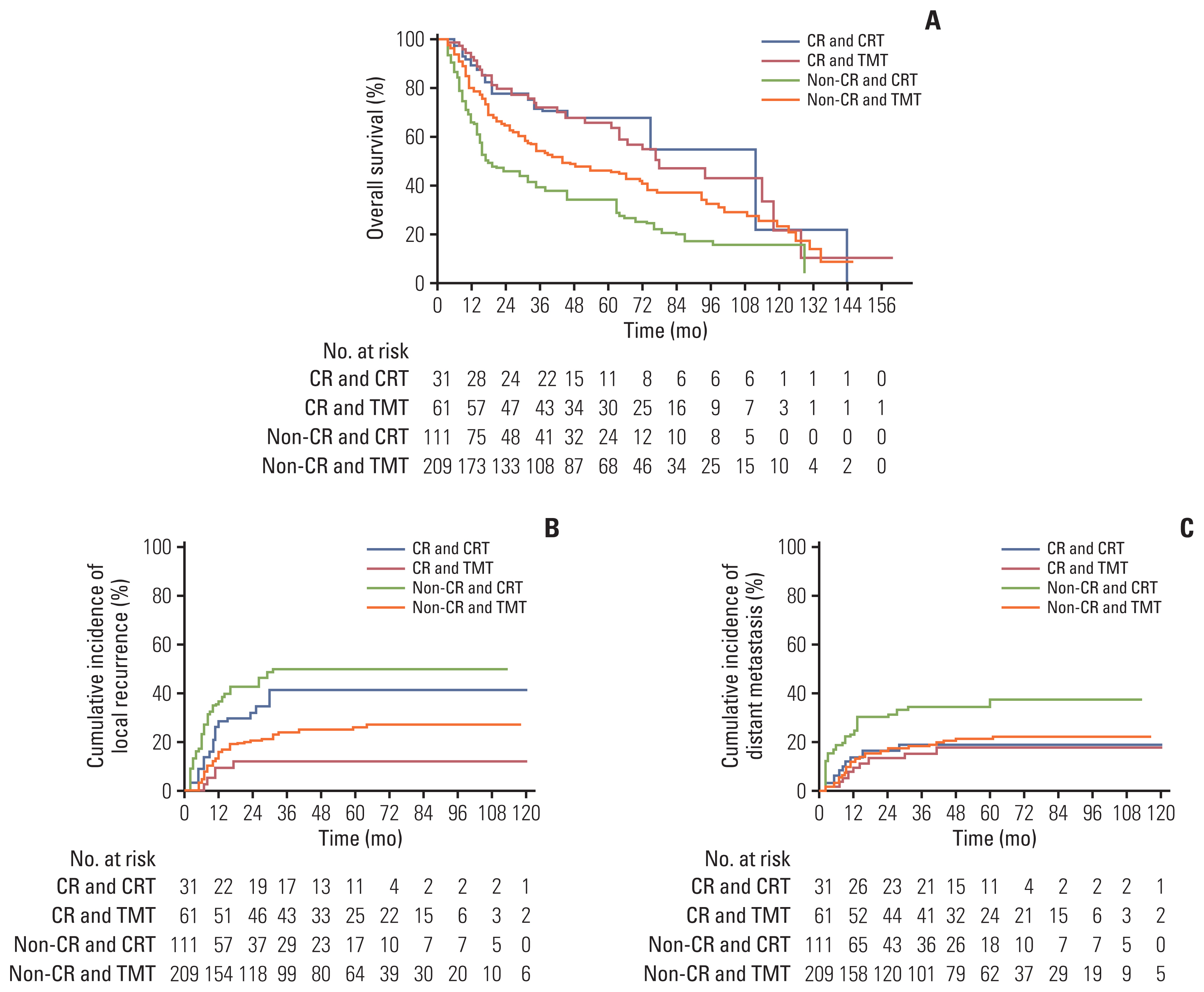
The median survival time was 64 and 32 months in the TMT (n=270) and CRT (n=142) groups, respectively (p < 0.001). After IPTW, the median overall survival (OS) remained significantly higher in the TMT group than in the CRT group (61 months vs. 32 months, p=0.016). Moreover, the TMT group showed a better local recurrence-free rate (LRFR, p < 0.001) and distant metastasis-free rate (p=0.007). In the subgroup of patients with clinical complete response (cCR), the OS was not significantly different between the two groups, both before and after IPTW adjustment (p=0.35 and p=0.93). However, among non-cCR patients, the OS was significantly higher in the TMT group (64% vs. 45%, p < 0.001).
Conclusion
In patients with locally advanced ESCC, TMT was superior to CRT in terms of OS and LRFR. Such difference was more prominent in the non-cCR subgroup. In patients who achieved cCR, esophagectomy was effective in improving LRFR but not OS, suggesting that esophagectomy may be omitted in complete responders.
Keyword
Figure
Reference
-
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68:394–424.2. van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012; 366:2074–84.3. Sjoquist KM, Burmeister BH, Smithers BM, Zalcberg JR, Simes RJ, Barbour A, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol. 2011; 12:681–92.4. Shapiro J, van Lanschot JJB, Hulshof M, van Hagen P, van Berge Henegouwen MI, Wijnhoven BP, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015; 16:1090–8.5. Stahl M, Stuschke M, Lehmann N, Meyer HJ, Walz MK, Seeber S, et al. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol. 2005; 23:2310–7.6. Bedenne L, Michel P, Bouche O, Milan C, Mariette C, Conroy T, et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol. 2007; 25:1160–8.7. Dubecz A, Sepesi B, Salvador R, Polomsky M, Watson TJ, Raymond DP, et al. Surgical resection for locoregional esophageal cancer is underutilized in the United States. J Am Coll Surg. 2010; 211:754–61.8. Takeuchi H, Miyata H, Gotoh M, Kitagawa Y, Baba H, Kimura W, et al. A risk model for esophagectomy using data of 5354 patients included in a Japanese nationwide web-based database. Ann Surg. 2014; 260:259–66.9. Piessen G, Messager M, Mirabel X, Briez N, Robb WB, Adenis A, et al. Is there a role for surgery for patients with a complete clinical response after chemoradiation for esophageal cancer? An intention-to-treat case-control study. Ann Surg. 2013; 258:793–9.10. Castoro C, Scarpa M, Cagol M, Alfieri R, Ruol A, Cavallin F, et al. Complete clinical response after neoadjuvant chemoradiotherapy for squamous cell cancer of the thoracic oesophagus: is surgery always necessary? J Gastrointest Surg. 2013; 17:1375–81.11. Chao YK, Tseng CK, Wen YW, Liu YH, Wan YL, Chiu CT, et al. Using pretreatment tumor depth and length to select esophageal squamous cell carcinoma patients for nonoperative treatment after neoadjuvant chemoradiotherapy. Ann Surg Oncol. 2013; 20:3000–8.12. Jeong Y, Kim JH, Kim SB, Yoon DH, Park SI, Kim YH, et al. Role of surgical resection in complete responders on FDG-PET after chemoradiotherapy for locally advanced esophageal squamous cell carcinoma. J Surg Oncol. 2014; 109:472–7.13. Yang H, Liu H, Chen Y, Zhu C, Fang W, Yu Z, et al. Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (NEOCRTEC5010): a phase III multicenter, randomized, open-label clinical trial. J Clin Oncol. 2018; 36:2796–803.14. Xi M, Yang Y, Zhang L, Yang H, Merrell KW, Hallemeier CL, et al. Multi-institutional analysis of recurrence and survival after neoadjuvant chemoradiotherapy of esophageal cancer: impact of histology on recurrence patterns and outcomes. Ann Surg. 2019; 269:663–70.15. Markar S, Gronnier C, Duhamel A, Pasquer A, Thereaux J, du Rieu MC, et al. Salvage surgery after chemoradiotherapy in the management of esophageal cancer: is it a viable therapeutic option? J Clin Oncol. 2015; 33:3866–73.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A case of squamous cell carcinoma arising from ovarian mature cystic teratoma treated with adjuvant chemoradiation
- How to Achieve a Higher Pathologic Complete Response in Patients With Locally Advanced Rectal Cancer Who Receive Preoperative Chemoradiation Therapy
- Clinical value of routine serum squamous cell carcinoma antigen in follow-up of patients with locally advanced cervical cancer treated with radiation or chemoradiation
- Chemotherapy of Head and Neck Cancer
- A Case of Complete Response in Locally Advanced Vulvar Cancer after Concomitant Chemoradiation Therapy