Diabetes Metab J.
2022 Sep;46(5):689-700. 10.4093/dmj.2021.0183.
Comparison of Efficacy of Glimepiride, Alogliptin, and Alogliptin-Pioglitazone as the Initial Periods of Therapy in Patients with Poorly Controlled Type 2 Diabetes Mellitus: An Open-Label, Multicenter, Randomized, Controlled Study
- Affiliations
-
- 1Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, Korea
- 2Department of Internal Medicine, Kyung Hee University Hospital at Gangdong, College of Medicine, Kyung Hee University, Seoul, Korea
- 3Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 4Department of Internal Medicine, CHA Bundang Medical Center, CHA University, Seongnam, Korea
- 5Department of Internal Medicine, Inje University Ilsan Paik Hospital, College of Medicine, Inje University, Goyang, Korea
- 6Department of Internal Medicine, Soonchunhyang University Cheonan Hospital, Cheonan, Korea
- 7Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea
- 8Department of Endocrinology and Metabolism, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea
- 9Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
- KMID: 2533638
- DOI: http://doi.org/10.4093/dmj.2021.0183
Abstract
- Background
The choice of an optimal oral hypoglycemic agent in the initial treatment periods for type 2 diabetes mellitus (T2DM) patients remains difficult and deliberate. We compared the efficacy and safety of glimepiride (GLIM), alogliptin (ALO), and alogliptin-pioglitazone (ALO-PIO) in poorly controlled T2DM patients with drug-naïve or metformin failure.
Methods
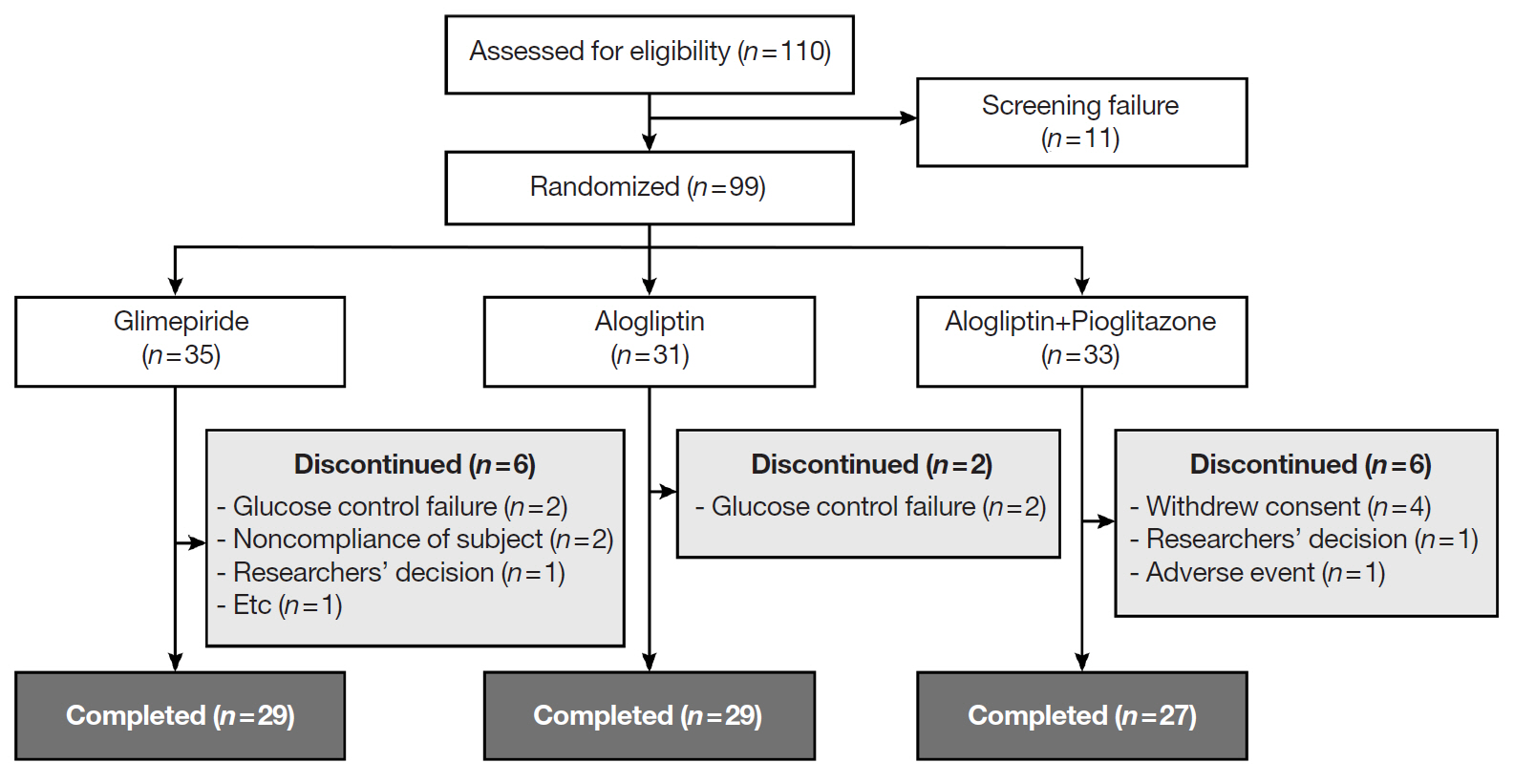
In this three-arm, multicenter, open-label, randomized, controlled trial, poorly controlled T2DM patients were randomized to receive GLIM (n=35), ALO (n=31), or ALO-PIO (n=33) therapy for 24 weeks. The primary endpoint was change in the mean glycosylated hemoglobin (HbA1c) levels at week 24 from baseline. Secondary endpoints were changes in HbA1c level at week 12 from baseline, fasting plasma glucose (FPG) levels, lipid profiles at weeks 12 and 24, and parameters of glycemic variability, assessed by continuous glucose monitoring for 24 weeks.
Results
At weeks 12 and 24, the ALO-PIO group showed significant reduction in HbA1c levels compared to the ALO group (–0.96%±0.17% vs. –0.37%±0.17% at week 12; –1.13%±0.19% vs. –0.18%±0.2% at week 24). The ALO-PIO therapy caused greater reduction in FPG levels and significant increase in high-density lipoprotein cholesterol levels at weeks 12 and 24 than the ALO therapy. Compared to low-dose GLIM therapy, ALO-PIO therapy showed greater improvement in glycemic variability. The adverse events were similar among the three arms.
Conclusion
ALO-PIO combination therapy during the early period exerts better glycemic control than ALO monotherapy and excellency in glycemic variability than low-dose sulfonylurea therapy in uncontrolled, drug-naïve or metformin failed T2DM patients.
Figure
Reference
-
1. Kahn SE. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia. 2003; 46:3–19.
Article2. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-Year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008; 359:1577–89.
Article3. Koliaki C, Doupis J. Incretin-based therapy: a powerful and promising weapon in the treatment of type 2 diabetes mellitus. Diabetes Ther. 2011; 2:101–21.
Article4. Deacon CF. Alogliptin, a potent and selective dipeptidyl peptidase-IV inhibitor for the treatment of type 2 diabetes. Curr Opin Investig Drugs. 2008; 9:402–13.5. DeFronzo RA, Eldor R, Abdul-Ghani M. Pathophysiologic approach to therapy in patients with newly diagnosed type 2 diabetes. Diabetes Care. 2013; 36(Suppl 2):S127–38.
Article6. Cahn A, Cefalu WT. Clinical considerations for use of initial combination therapy in type 2 diabetes. Diabetes Care. 2016; 39(Suppl 2):S137–45.
Article7. Matthews DR, Paldanius PM, Proot P, Chiang Y, Stumvoll M, Del Prato S, et al. Glycaemic durability of an early combination therapy with vildagliptin and metformin versus sequential metformin monotherapy in newly diagnosed type 2 diabetes (VERIFY): a 5-year, multicentre, randomised, double-blind trial. Lancet. 2019; 394:1519–29.
Article8. DeFronzo RA, Burant CF, Fleck P, Wilson C, Mekki Q, Pratley RE. Efficacy and tolerability of the DPP-4 inhibitor alogliptin combined with pioglitazone, in metformin-treated patients with type 2 diabetes. J Clin Endocrinol Metab. 2012; 97:1615–22.
Article9. Derosa G, Maffioli P. Peroxisome proliferator-activated receptor-γ (PPAR-γ) agonists on glycemic control, lipid profile and cardiovascular risk. Curr Mol Pharmacol. 2012; 5:272–81.
Article10. Soccio RE, Chen ER, Lazar MA. Thiazolidinediones and the promise of insulin sensitization in type 2 diabetes. Cell Metab. 2014; 20:573–91.
Article11. Ohara M, Kohata Y, Nagaike H, Koshibu M, Gima H, Hiromura M, et al. Association of glucose and blood pressure variability on oxidative stress in patients with type 2 diabetes mellitus and hypertension: a cross-sectional study. Diabetol Metab Syndr. 2019; 11:29.
Article12. Zhou Z, Sun B, Huang S, Zhu C, Bian M. Glycemic variability: adverse clinical outcomes and how to improve it? Cardiovasc Diabetol. 2020; 19:102.
Article13. Rodrigues R, de Medeiros LA, Cunha LM, Garrote-Filho MDS, Bernardino Neto M, Jorge PT, et al. Correlations of the glycemic variability with oxidative stress and erythrocytes membrane stability in patients with type 1 diabetes under intensive treatment. Diabetes Res Clin Pract. 2018; 144:153–60.
Article14. Sola D, Rossi L, Schianca GP, Maffioli P, Bigliocca M, Mella R, et al. Sulfonylureas and their use in clinical practice. Arch Med Sci. 2015; 11:840–8.15. Bailey T, Bode BW, Christiansen MP, Klaff LJ, Alva S. The performance and usability of a factory-calibrated flash glucose monitoring system. Diabetes Technol Ther. 2015; 17:787–94.
Article16. Defronzo RA. Banting lecture: from the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009; 58:773–95.17. Triplitt C, Cersosimo E, DeFronzo RA. Pioglitazone and alogliptin combination therapy in type 2 diabetes: a pathophysiologically sound treatment. Vasc Health Risk Manag. 2010; 6:671–90.18. Yki-Jarvinen H. Thiazolidinediones. N Engl J Med. 2004; 351:1106–18.
Article19. Rosenstock J, Zinman B. Dipeptidyl peptidase-4 inhibitors and the management of type 2 diabetes mellitus. Curr Opin Endocrinol Diabetes Obes. 2007; 14:98–107.
Article20. Moritoh Y, Takeuchi K, Asakawa T, Kataoka O, Odaka H. Combining a dipeptidyl peptidase-4 inhibitor, alogliptin, with pioglitazone improves glycaemic control, lipid profiles and beta-cell function in db/db mice. Br J Pharmacol. 2009; 157:415–26.21. Moritoh Y, Takeuchi K, Asakawa T, Kataoka O, Odaka H. The dipeptidyl peptidase-4 inhibitor alogliptin in combination with pioglitazone improves glycemic control, lipid profiles, and increases pancreatic insulin content in ob/ob mice. Eur J Pharmacol. 2009; 602:448–54.22. Kawashima S, Matsuoka TA, Kaneto H, Tochino Y, Kato K, Yamamoto K, et al. Effect of alogliptin, pioglitazone and glargine on pancreatic β-cells in diabetic db/db mice. Biochem Biophys Res Commun. 2011; 404:534–40.23. Rosenstock J, Inzucchi SE, Seufert J, Fleck PR, Wilson CA, Mekki Q. Initial combination therapy with alogliptin and pioglitazone in drug-naive patients with type 2 diabetes. Diabetes Care. 2010; 33:2406–8.24. Flory JH, Small DS, Cassano PA, Brillon DJ, Mushlin AI, Hennessy S. Comparative effectiveness of oral diabetes drug combinations in reducing glycosylated hemoglobin. J Comp Eff Res. 2014; 3:29–39.
Article25. Kim YM, Cha BS, Kim DJ, Choi SH, Kim SK, Ahn CW, et al. Predictive clinical parameters for therapeutic efficacy of rosiglitazone in Korean type 2 diabetes mellitus. Diabetes Res Clin Pract. 2005; 67:43–52.
Article26. Park SE, Lee BW, Kim JH, Lee WJ, Cho JH, Jung CH, et al. Effect of gemigliptin on glycaemic variability in patients with type 2 diabetes (STABLE study). Diabetes Obes Metab. 2017; 19:892–6.
Article27. Kim NH, Kim DL, Kim KJ, Kim NH, Choi KM, Baik SH, et al. Effects of vildagliptin or pioglitazone on glycemic variability and oxidative stress in patients with type 2 diabetes inadequately controlled with metformin monotherapy: a 16-week, randomised, open label, pilot study. Endocrinol Metab (Seoul). 2017; 32:241–7.
Article28. Rosenstock J, Wilson C, Fleck P. Alogliptin versus glipizide monotherapy in elderly type 2 diabetes mellitus patients with mild hyperglycaemia: a prospective, double-blind, randomized, 1-year study. Diabetes Obes Metab. 2013; 15:906–14.
Article29. Mishriky BM, Cummings DM, Tanenberg RJ. The efficacy and safety of DPP4 inhibitors compared to sulfonylureas as add-on therapy to metformin in patients with type 2 diabetes: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2015; 109:378–88.
Article30. Goldberg RB, Kendall DM, Deeg MA, Buse JB, Zagar AJ, Pinaire JA, et al. A comparison of lipid and glycemic effects of pioglitazone and rosiglitazone in patients with type 2 diabetes and dyslipidemia. Diabetes Care. 2005; 28:1547–54.
Article31. Florkowski CM. Management of co-existing diabetes mellitus and dyslipidemia: defining the role of thiazolidinediones. Am J Cardiovasc Drugs. 2002; 2:15–21.32. Snyder HS, Sakaan SA, March KL, Siddique O, Cholankeril R, Cummings CD, et al. Non-alcoholic fatty liver disease: a review of anti-diabetic pharmacologic therapies. J Clin Transl Hepatol. 2018; 6:168–74.
Article33. Musso G, Cassader M, Paschetta E, Gambino R. Thiazolidinediones and advanced liver fibrosis in nonalcoholic steatohepatitis: a meta-analysis. JAMA Intern Med. 2017; 177:633–40.
Article34. Mashitani T, Noguchi R, Okura Y, Namisaki T, Mitoro A, Ishii H, et al. Efficacy of alogliptin in preventing non-alcoholic fatty liver disease progression in patients with type 2 diabetes. Biomed Rep. 2016; 4:183–7.
Article35. Kaku K, Katou M, Igeta M, Ohira T, Sano H. Efficacy and safety of pioglitazone added to alogliptin in Japanese patients with type 2 diabetes mellitus: a multicentre, randomized, double-blind, parallel-group, comparative study. Diabetes Obes Metab. 2015; 17:1198–201.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Efficacy and Safety of Pioglitazone versus Glimepiride after Metformin and Alogliptin Combination Therapy: A Randomized, Open-Label, Multicenter, Parallel-Controlled Study
- Comparison of Vildagliptin-Metformin and Glimepiride-Metformin Treatments in Type 2 Diabetic Patients
- Efficacy and Safety of Glimepiride: A Novel Sulfonylurea Drug compared with Gliclazide in the Treatment of Type 2 Diabetes Mellitus: an Open , Randomized Comparative Multi - Center Clinical Study
- Letter: Efficacy and Safety of Biphasic Insulin Aspart 30/70 in Type 2 Diabetes Suboptimally Controlled on Oral Antidiabetic Therapy in Korea: A Multicenter, Open-Label, Single-Arm Study (Diabetes Metab J 2013;37:117-24)
- Effects of Vildagliptin or Pioglitazone on Glycemic Variability and Oxidative Stress in Patients with Type 2 Diabetes Inadequately Controlled with Metformin Monotherapy: A 16-Week, Randomised, Open Label, Pilot Study