Int J Stem Cells.
2022 Aug;15(3):334-345. 10.15283/ijsc22044.
Modulation of Osteogenic Differentiation of Adipose-Derived Stromal Cells by Co-Treatment with 3, 4’-Dihydroxyflavone, U0126, and N-Acetyl Cysteine
- Affiliations
-
- 1Department of Stem Cell and Regenerative Biotechnology and Incurable Disease Animal Model and Stem Cell Institute (IDASI), Konkuk University, Seoul, Korea
- 2Human Molecular Cytogenetics and Stem Cell Laboratory, Department of Human Genetics and Molecular Biology, Bharathiar University, Coimbatore, India
- KMID: 2532407
- DOI: http://doi.org/10.15283/ijsc22044
Abstract
- Background and Objectives
Flavonoids form the largest group of plant phenols and have various biological and pharma-cological activities. In this study, we investigated the effect of a flavonoid, 3, 4’-dihydroxyflavone (3, 4’-DHF) on osteogenic differentiation of equine adipose-derived stromal cells (eADSCs).
Methods and Results
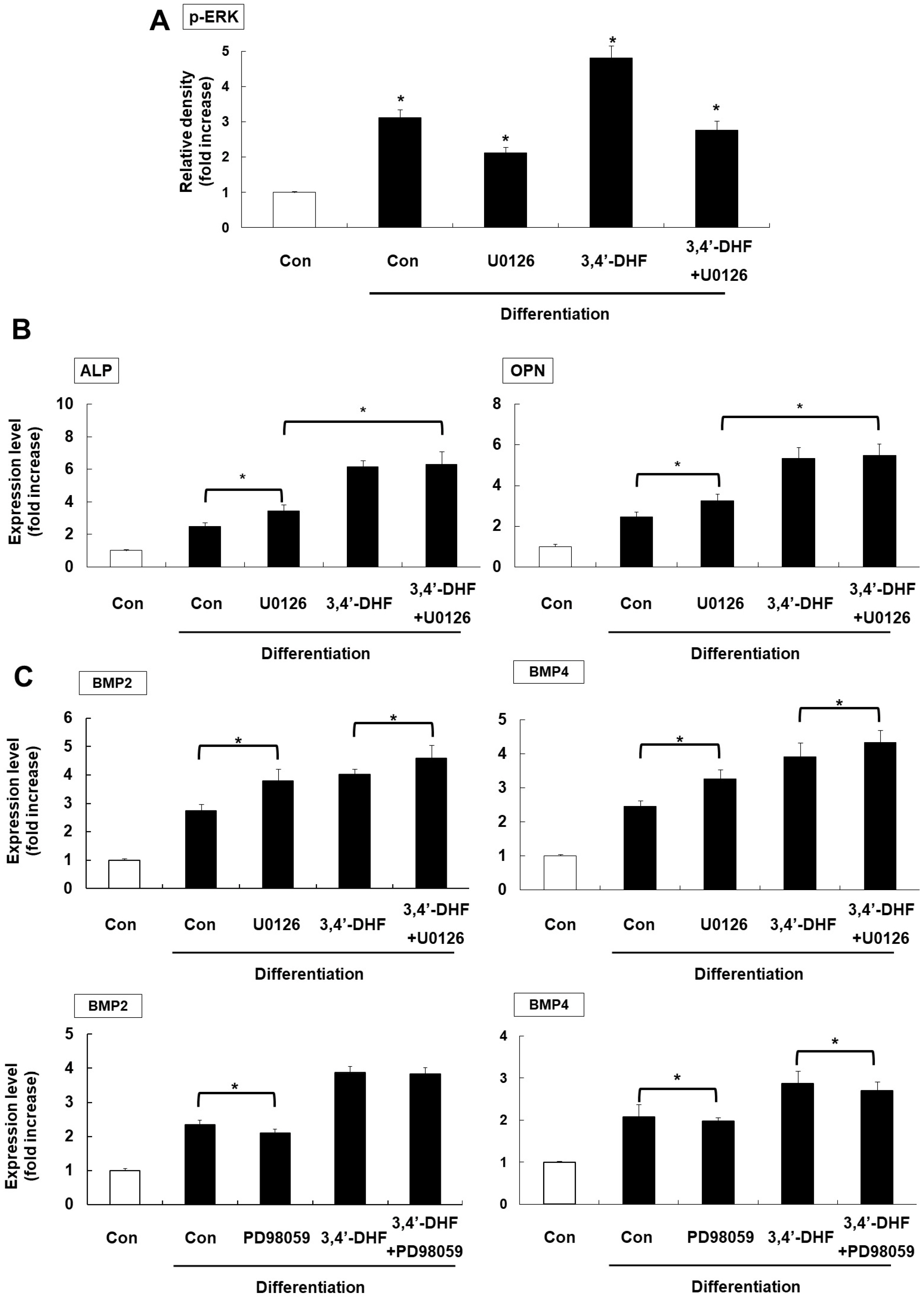
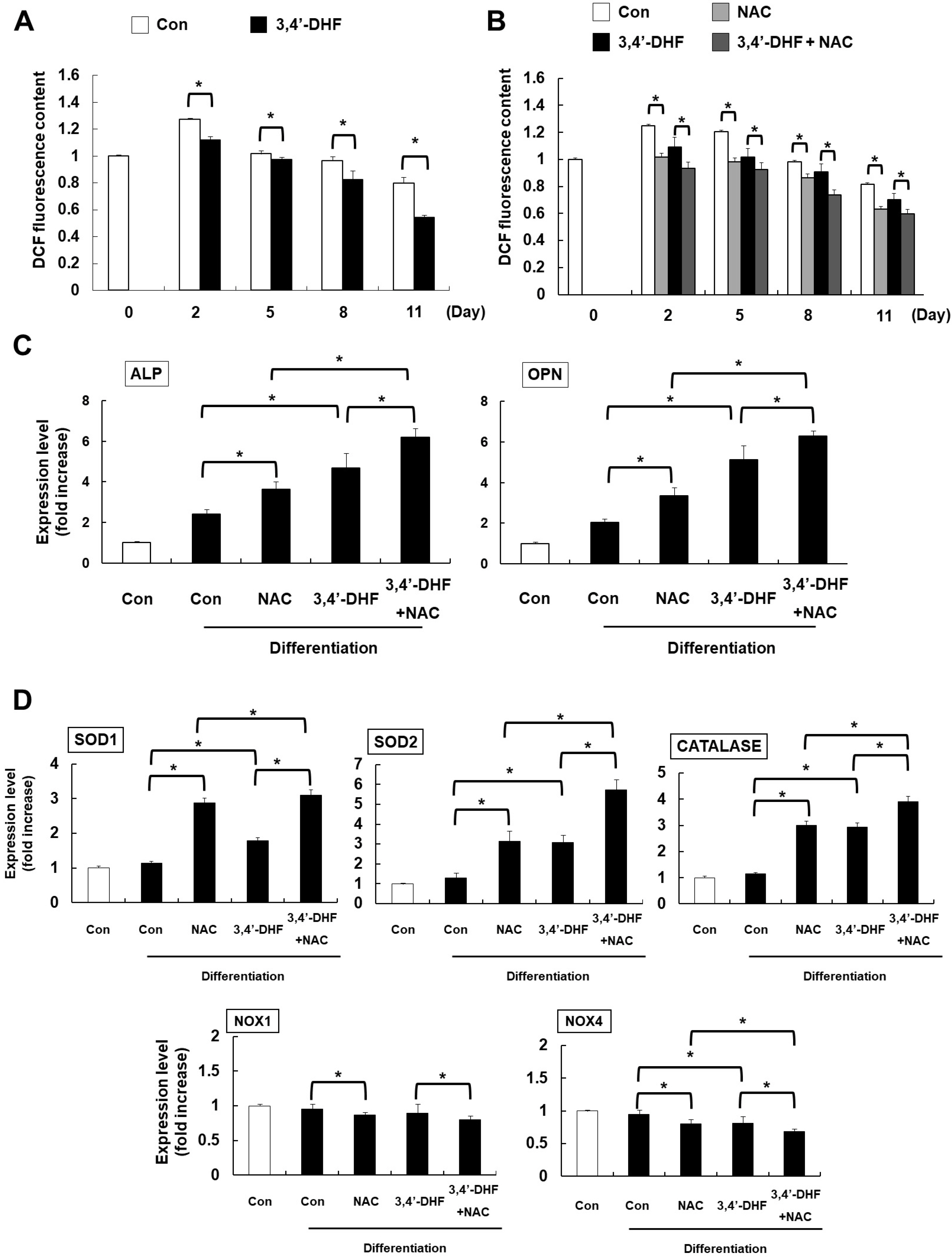
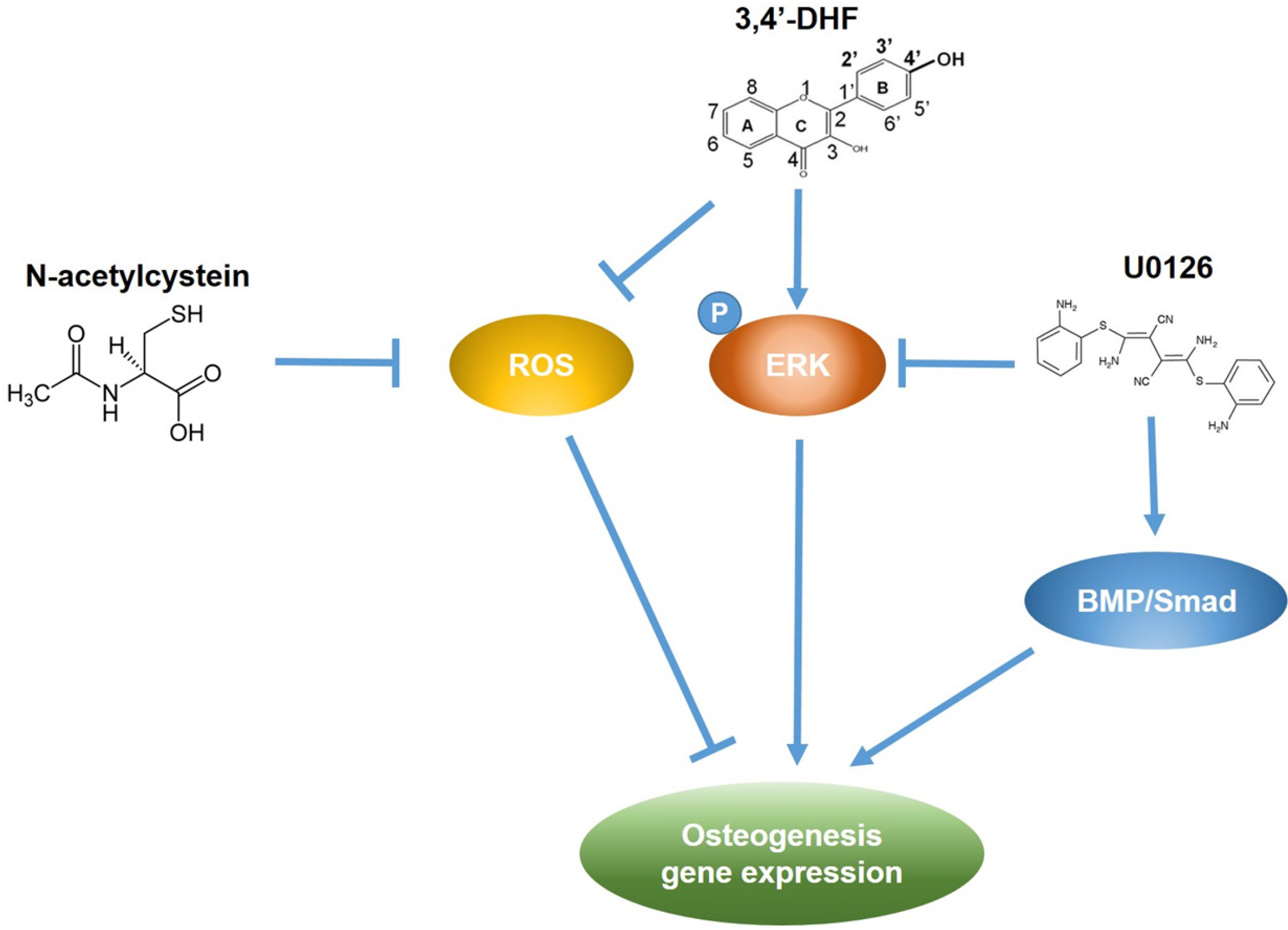
Treatment of 3, 4’-DHF led to increased osteogenic differentiation of eADSCs by increasing phosphorylation of ERK and modulating Reactive Oxygen Species (ROS) generation. Although PD98059, an ERK inhibitor, suppressed osteogenic differentiation, another ERK inhibitor, U0126, apparently increased osteogenic differentiation of the 3, 4’-DHF-treated eADSCs, which may indicate that the effect of U0126 on bone morphogenetic protein signaling is involved in the regulation of 3, 4’-DHF in osteogenic differentiation of eADSCs. We revealed that 3, 4’-DHF could induce osteogenic differentiation of eADSCs by suppressing ROS generation and co-treatment of 3, 4’-DHF, U0126, and/or N-acetyl cysteine (NAC) resulted in the additive enhancement of osteogenic differentiation of eADSCs.
Conclusions
Our results showed that co-treatment of 3, 4’-DHF, U0126, and/or NAC cumulatively regulated osteo-genesis in eADSCs, suggesting that 3, 4’-DHF, a flavonoid, can provide a novel approach to the treatment of osteoporosis and can provide potential therapeutic applications in therapeutics and regenerative medicine for human and companion animals.
Keyword
Figure
Reference
-
References
1. Arai M, Shibata Y, Pugdee K, Abiko Y, Ogata Y. 2007; Effects of reactive oxygen species (ROS) on antioxidant system and osteoblastic differentiation in MC3T3-E1 cells. IUBMB Life. 59:27–33. DOI: 10.1080/15216540601156188. PMID: 17365177. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=33847046732&origin=inward.2. Maruotti N, Corrado A, Neve A, Cantatore FP. 2012; Bisphos-phonates: effects on osteoblast. Eur J Clin Pharmacol. 68:1013–1018. DOI: 10.1007/s00228-012-1216-7. PMID: 22318756. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84864276811&origin=inward.3. Huang Q, Gao B, Jie Q, Wei BY, Fan J, Zhang HY, Zhang JK, Li XJ, Shi J, Luo ZJ, Yang L, Liu J. 2014; Ginsenoside-Rb2 displays anti-osteoporosis effects through reducing oxidative damage and bone-resorbing cytokines during osteo-genesis. Bone. 66:306–314. DOI: 10.1016/j.bone.2014.06.010. PMID: 24933344. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84904569485&origin=inward.4. Kular J, Tickner J, Chim SM, Xu J. 2012; An overview of the regulation of bone remodelling at the cellular level. Clin Biochem. 45:863–873. DOI: 10.1016/j.clinbiochem.2012.03.021. PMID: 22465238. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84863875184&origin=inward.5. Zhai YK, Guo XY, Ge BF, Zhen P, Ma XN, Zhou J, Ma HP, Xian CJ, Chen KM. 2014; Icariin stimulates the osteogenic differentiation of rat bone marrow stromal cells via activating the PI3K-AKT-eNOS-NO-cGMP-PKG. Bone. 66:189–198. DOI: 10.1016/j.bone.2014.06.016. PMID: 24956021. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84903639742&origin=inward.6. Vidal MA, Kilroy GE, Lopez MJ, Johnson JR, Moore RM, Gimble JM. 2007; Characterization of equine adipose tissue-derived stromal cells: adipogenic and osteogenic capacity and comparison with bone marrow-derived mesenchymal stromal cells. Vet Surg. 36:613–622. DOI: 10.1111/j.1532-950X.2007.00313.x. PMID: 17894587. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=34748850083&origin=inward.7. Mathieu PS, Loboa EG. 2012; Cytoskeletal and focal adhesion influences on mesenchymal stem cell shape, mechanical properties, and differentiation down osteogenic, adipogenic, and chondrogenic pathways. Tissue Eng Part B Rev. 18:436–444. DOI: 10.1089/ten.teb.2012.0014. PMID: 22741572. PMCID: PMC3495119. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84869014728&origin=inward.8. Braun J, Hack A, Weis-Klemm M, Conrad S, Treml S, Kohler K, Walliser U, Skutella T, Aicher WK. 2010; Evaluation of the osteogenic and chondrogenic differentiation capacities of equine adipose tissue-derived mesenchymal stem cells. Am J Vet Res. 71:1228–1236. DOI: 10.2460/ajvr.71.10.1228. PMID: 20919912. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=77958607412&origin=inward.9. Zhang L, Su P, Xu C, Chen C, Liang A, Du K, Peng Y, Huang D. 2010; Melatonin inhibits adipogenesis and enhances osteogenesis of human mesenchymal stem cells by suppressing PPARγ expression and enhancing Runx2 expression. J Pineal Res. 49:364–372. DOI: 10.1111/j.1600-079X.2010.00803.x. PMID: 20738756. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=78349247846&origin=inward.10. Whitworth DJ, Ovchinnikov DA, Sun J, Fortuna PR, Wolvetang EJ. 2014; Generation and characterization of leukemia inhibitory factor-dependent equine induced pluripotent stem cells from adult dermal fibroblasts. Stem Cells Dev. 23:1515–1523. DOI: 10.1089/scd.2013.0461. PMID: 24555755. PMCID: PMC4066230. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84903183230&origin=inward.11. Sharma R, Livesey MR, Wyllie DJ, Proudfoot C, Whitelaw CB, Hay DC, Donadeu FX. 2014; Generation of functional neurons from feeder-free, keratinocyte-derived equine induced pluripotent stem cells. Stem Cells Dev. 23:1524–1534. DOI: 10.1089/scd.2013.0565. PMID: 24548115. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84903214981&origin=inward.12. Nagy K, Sung HK, Zhang P, Laflamme S, Vincent P, Agha-Mohammadi S, Woltjen K, Monetti C, Michael IP, Smith LC, Nagy A. 2011; Induced pluripotent stem cell lines derived from equine fibroblasts. Stem Cell Rev Rep. 7:693–702. Erratum in: Stem Cell Rev 2012;8:546. DOI: 10.1007/s12015-011-9239-5. PMID: 21347602. PMCID: PMC3137777. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=79960290466&origin=inward.13. Arens AM, Barr B, Puchalski SM, Poppenga R, Kulin RM, Anderson J, Stover SM. 2011; Osteoporosis associated with pulmonary silicosis in an equine bone fragility syndrome. Vet Pathol. 48:593–615. DOI: 10.1177/0300985810385151. PMID: 21097716. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=79955847838&origin=inward.14. de Mattos Carvalho A, Alves ALG, de Oliveira PGG, Cisneros Álvarez LE, Amorim RL, Hussni CA, Deffune E. 2011; Use of adipose tissue-derived mesenchymal stem cells for experimental tendinitis therapy in equines. J Equine Vet Sci. 31:26–34. DOI: 10.1016/j.jevs.2010.11.014. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=78650987170&origin=inward.15. Nixon AJ, Dahlgren LA, Haupt JL, Yeager AE, Ward DL. 2008; Effect of adipose-derived nucleated cellfractions on tendon repair in horses with collagenase-induced tendinitis. Am J Vet Res. 69:928–937. DOI: 10.2460/ajvr.69.7.928. PMID: 18593247. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=48049104541&origin=inward.16. Carvalho Ade M, Badial PR, Álvarez LE, Yamada AL, Borges AS, Deffune E, Hussni CA, Garcia Alves AL. 2013; Equine tendonitis therapy using mesenchymal stem cells and platelet concentrates: a randomized controlled trial. Stem Cell Res Ther. 4:85. DOI: 10.1186/scrt236. PMID: 23876512. PMCID: PMC3854756. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84880335347&origin=inward.17. Conze P, van Schie HT, van Weeren R, Staszyk C, Conrad S, Skutella T, Hopster K, Rohn K, Stadler P, Geburek F. 2014; Effect of autologous adipose tissue-derived mesenchymal stem cells on neovascularization of artificial equine tendon lesions. Regen Med. 9:743–757. DOI: 10.2217/rme.14.55. PMID: 25431911. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84913616782&origin=inward.18. Zhang JF, Li G, Chan CY, Meng CL, Lin MC, Chen YC, He ML, Leung PC, Kung HF. 2010; Flavonoids of Herba Epimedii regulate osteogenesis of human mesenchymal stem cells through BMP and Wnt/beta-catenin signaling pathway. Mol Cell Endocrinol. 314:70–74. DOI: 10.1016/j.mce.2009.08.012. PMID: 19703516. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=70350532232&origin=inward.19. Lee ER, Kang YJ, Kim JH, Lee HT, Cho SG. 2005; Modulation of apoptosis in HaCaT keratinocytes via differential regulation of ERK signaling pathway by flavonoids. J Biol Chem. 280:31498–31507. DOI: 10.1074/jbc.M505537200. PMID: 16014620. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=24744448676&origin=inward.20. Lee ER, Kim JH, Kang YJ, Cho SG. 2007; The anti-apoptotic and anti-oxidant effect of eriodictyol on UV-induced apoptosis in keratinocytes. Biol Pharm Bull. 30:32–37. DOI: 10.1248/bpb.30.32. PMID: 17202655. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=33846076220&origin=inward.21. Lee ER, Kim JH, Choi HY, Jeon K, Cho SG. 2011; Cytoprotective effect of eriodictyol in UV-irradiated keratinocytes via phosphatase-dependent modulation of both the p38 MAPK and Akt signaling pathways. Cell Physiol Biochem. 27:513–524. DOI: 10.1159/000329973. PMID: 21691069. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=79959432364&origin=inward.22. Lee ER, Kim JY, Kang YJ, Ahn JY, Kim JH, Kim BW, Choi HY, Jeong MY, Cho SG. 2006; Interplay between PI3K/Akt and MAPK signaling pathways in DNA-damaging drug-induced apoptosis. Biochim Biophys Acta. 1763:958–968. DOI: 10.1016/j.bbamcr.2006.06.006. PMID: 16905201. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=33748464742&origin=inward.23. Kim BW, Lee ER, Min HM, Jeong HS, Ahn JY, Kim JH, Choi HY, Choi H, Kim EY, Park SP, Cho SG. 2008; Sustained ERK activation is involved in the kaempferol-induced apoptosis of breast cancer cells and is more evident under 3-D culture condition. Cancer Biol Ther. 7:1080–1089. DOI: 10.4161/cbt.7.7.6164. PMID: 18443432. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=47949120075&origin=inward.24. Han D, Kim HJ, Choi HY, Kim B, Yang G, Han J, Dayem AA, Lee HR, Kim JH, Lee KM, Jeong KS, Do SH, Cho SG. 2015; 3,2'-dihydroxyflavone-treated pluripotent stem cells show enhanced proliferation, pluripotency marker expression, and neuroprotective properties. Cell Transplant. 24:1511–1532. DOI: 10.3727/096368914X683511. PMID: 25198120. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84938894999&origin=inward.25. Lee KS, Kim EY, Jeon K, Cho SG, Han YJ, Yang BC, Lee SS, Ko MS, Riu KJ, Lee HT, Park SP. 2011; 3,4-Dihydroxyflavone acts as an antioxidant and antiapoptotic agent to support bovine embryo development in vitro. J Reprod Dev. 57:127–134. DOI: 10.1262/jrd.10-029A. PMID: 21071889. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=79952954930&origin=inward.26. Xu L, Liu Y, Hou Y, Wang K, Wong Y, Lin S, Li G. 2015; U0126 promotes osteogenesis of rat bone-marrow-derived mesenchymal stem cells by activating BMP/Smad signaling pathway. Cell Tissue Res. 359:537–545. DOI: 10.1007/s00441-014-2025-3. PMID: 25363751. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84925538758&origin=inward.27. Han J, Choi HY, Dayem AA, Kim K, Yang G, Won J, Do SH, Kim JH, Jeong KS, Cho SG. 2017; Regulation of adipogenesis through differential modulation of ROS and kinase signaling pathways by 3,4'-dihydroxyflavone treatment. J Cell Biochem. 118:1065–1077. DOI: 10.1002/jcb.25681. PMID: 27579626. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85008500304&origin=inward.28. Panche AN, Diwan AD, Chandra SR. 2016; Flavonoids: an overview. J Nutr Sci. 5:e47. DOI: 10.1017/jns.2016.41. PMID: 28620474. PMCID: PMC5465813. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85007481080&origin=inward.29. Preethi Soundarya S, Sanjay V, Haritha Menon A, Dhivya S, Selvamurugan N. 2018; Effects of flavonoids incorporated biological macromolecules based scaffolds in bone tissue engineering. Int J Biol Macromol. 110:74–87. DOI: 10.1016/j.ijbiomac.2017.09.014. PMID: 28893682. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85029453032&origin=inward.30. Cheng SL, Shao JS, Charlton-Kachigian N, Loewy AP, Towler DA. 2003; MSX2 promotes osteogenesis and suppresses adipogenic differentiation of multipotent mesenchymal progenitors. J Biol Chem. 278:45969–45977. DOI: 10.1074/jbc.M306972200. PMID: 12925529. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0242664267&origin=inward.31. Hong G, Chen Z, Han X, Zhou L, Pang F, Wu R, Shen Y, He X, Hong Z, Li Z, He W, Wei Q. 2021; A novel RANKL-targeted flavonoid glycoside prevents osteoporosis through inhibiting NFATc1 and reactive oxygen species. Clin Transl Med. 11:e392. DOI: 10.1002/ctm2.392. PMID: 34047464. PMCID: PMC8140192. PMID: 569a434feee74b6d87223a0dacbd4589. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85107226036&origin=inward.32. Jiang J, Xiao S, Xu X, Ma H, Feng C, Jia X. 2018; Isomeric flavonoid aglycones derived from Epimedii Folium exerted different intensities in anti-osteoporosis through OPG/RANKL protein targets. Int Immunopharmacol. 62:277–286. DOI: 10.1016/j.intimp.2018.07.017. PMID: 30036771. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85050100519&origin=inward.33. Martiniakova M, Babikova M, Mondockova V, Blahova J, Kovacova V, Omelka R. 2022; The role of macronutrients, micronutrients and flavonoid polyphenols in the prevention and treatment of osteoporosis. Nutrients. 14:523. DOI: 10.3390/nu14030523. PMID: 35276879. PMCID: PMC8839902. PMID: 2ec37e55d87041e680aa39d79aaf757d. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85123290802&origin=inward.34. Laflamme C, Curt S, Rouabhia M. 2010; Epidermal growth factor and bone morphogenetic proteins upregulate osteoblast proliferation and osteoblastic markers and inhibit bone nodule formation. Arch Oral Biol. 55:689–701. DOI: 10.1016/j.archoralbio.2010.06.010. PMID: 20627196. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=78149358646&origin=inward.35. Marie PJ. 2012; Fibroblast growth factor signaling controlling bone formation: an update. Gene. 498:1–4. DOI: 10.1016/j.gene.2012.01.086. PMID: 22342254. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84858159772&origin=inward.36. Yu Y, Mu J, Fan Z, Lei G, Yan M, Wang S, Tang C, Wang Z, Yu J, Zhang G. 2012; Insulin-like growth factor 1 enhances the proliferation and osteogenic differentiation of human periodontal ligament stem cells via ERK and JNK MAPK pathways. Histochem Cell Biol. 137:513–525. DOI: 10.1007/s00418-011-0908-x. PMID: 22227802. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84862899040&origin=inward.37. Lee DH, Lim BS, Lee YK, Yang HC. 2006; Effects of hydrogen peroxide (H2O2) on alkaline phosphatase activity and matrix mineralization of odontoblast and osteoblast cell lines. Cell Biol Toxicol. 22:39–46. DOI: 10.1007/s10565-006-0018-z. PMID: 16463018. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=32144434247&origin=inward.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Coculture Effects on the Osteogenic Differentiation of Human Mesenchymal Stromal Cells
- The effect of growth factors on osteogenic differentiation of adipose tissue-derived stromal cells
- Osteogenic Differentiation of Human Adipose-derived Stem Cells within PLGA(Poly(D,L-lactic-co-glycolic acid)) Scaffold in the Nude Mouse
- A study on the osteogenic differentiation of adipose-derived adult stem cell
- MicroRNA-103a-3p controls proliferation and osteogenic differentiation of human adipose tissue-derived stromal cells