Int J Stem Cells.
2022 Aug;15(3):283-290. 10.15283/ijsc21179.
Development of a High-Yield Isolation Protocol Optimized for the Retrieval of Active Muscle Satellite Cells from Mouse Skeletal Muscle Tissue
- Affiliations
-
- 1Department of Animal Life Science, Kangwon National University, Chuncheon, Korea
- 2SCBIO Co., Ltd., Daejeon, Korea
- 3KustoGen Inc., Chuncheon, Korea
- 4Department of Applied Animal Science, Kangwon National University, Chuncheon, Korea
- KMID: 2532403
- DOI: http://doi.org/10.15283/ijsc21179
Abstract
- Background and Objectives
Difficulties often encountered in separating and purifying active muscle satellite cells (MSCs) from skeletal muscle tissues have limited the supply of cells for muscle therapy and artificial meat production. Here, we report an effective isolation protocol to economically and conveniently retrieve active MSCs from skeletal muscle tissues in mice.
Methods and Results
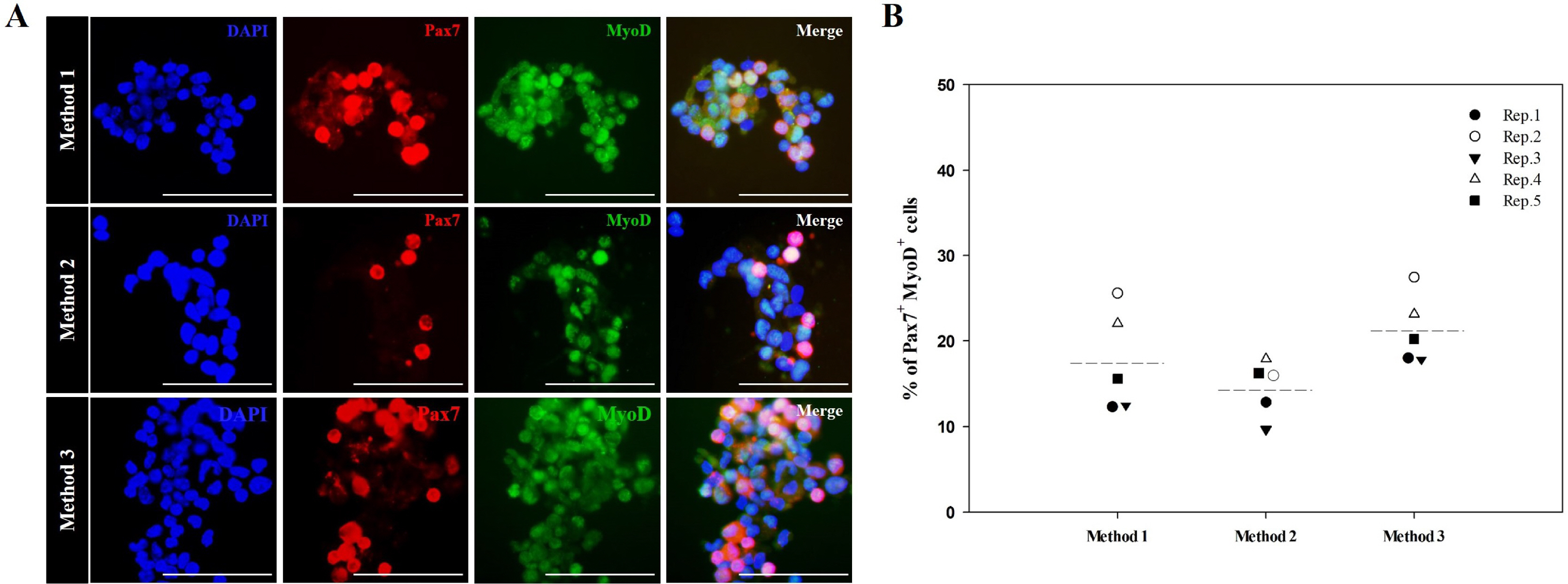
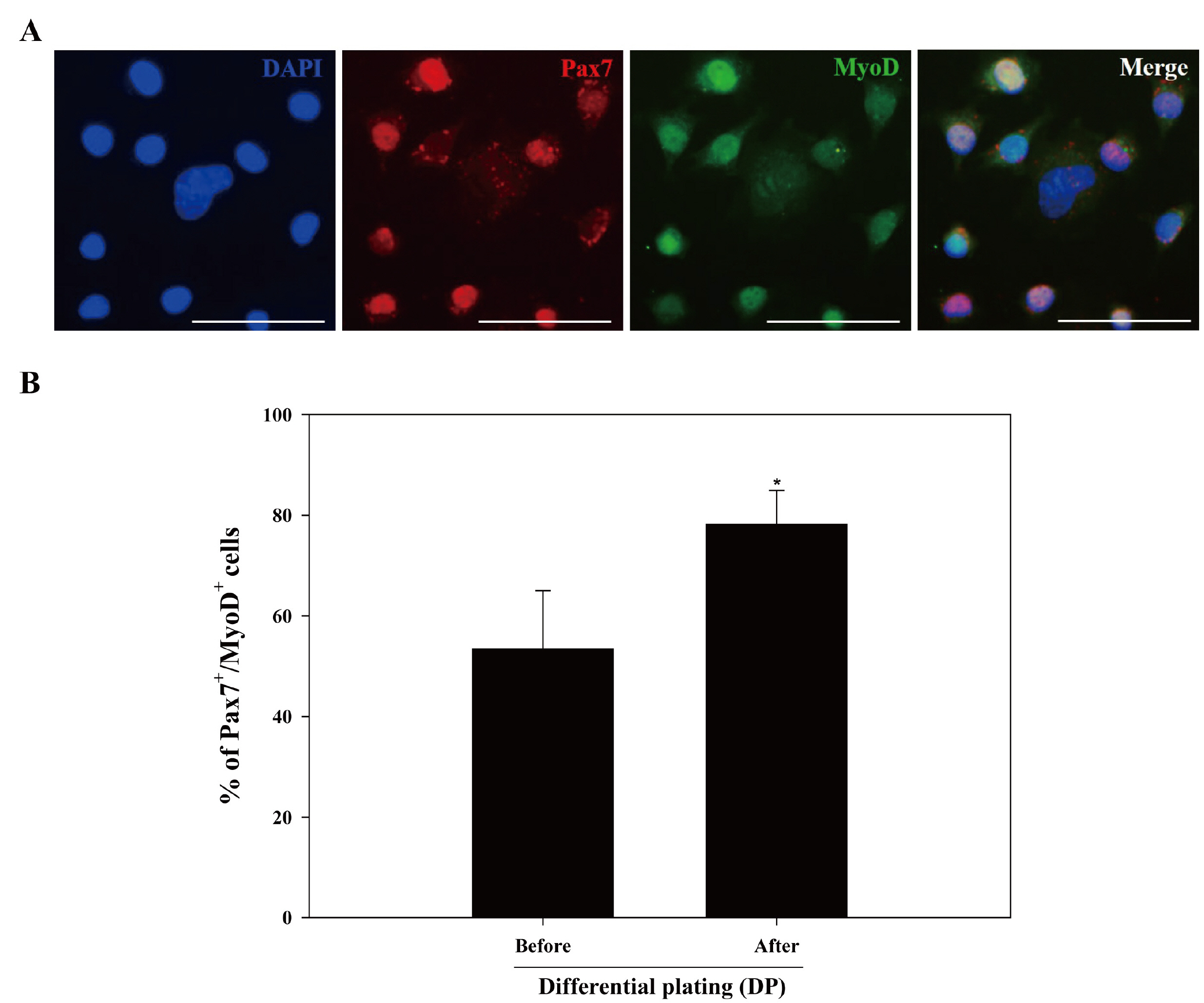
We optimized an enzyme-based tissue digestion protocol for isolating skeletal muscle-derived primary cell population having a large number of active MSCs and described a method of differential plating (DP) for improving purity of active MSCs from skeletal muscle-derived primary cell population. Then, the age of the mouse appropriate to the isolation of a large number of active MSCs was elucidated. The best isolation yield of active MSCs from mouse skeletal muscle tissues was induced by the application of DP method to the primary cell population harvested from skeletal muscle tissues of 2-week-old mice digested in 0.2% (w/v) collagenase type II for 30 min at 37℃ and then in 0.1% (w/v) pronase for 5 min at 37℃.
Conclusions
The protocol we developed not only facilitates the isolation of MSCs but also maximizes the retrieval of active MSCs. Our expectation is that this protocol will contribute to the development of original technologies essential for muscle therapy and artificial meat industrialization in the future.
Keyword
Figure
Reference
-
References
1. Chen W, Datzkiw D, Rudnicki MA. 2020; Satellite cells in ageing: use it or lose it. Open Biol. 10:200048. DOI: 10.1098/rsob.200048. PMID: 32428419. PMCID: PMC7276531. PMID: cfe5a68370ff46d6af0311c09747b508. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85084961672&origin=inward.2. Han WM, Anderson SE, Mohiuddin M, Barros D, Nakhai SA, Shin E, Amaral IF, Pêgo AP, García AJ, Jang YC. 2018; Synthetic matrix enhances transplanted satellite cell engraftment in dystrophic and aged skeletal muscle with comorbid trauma. Sci Adv. 4:eaar4008. DOI: 10.1126/sciadv.aar4008. PMID: 30116776. PMCID: PMC6093653. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85052200928&origin=inward.3. Cho CH, Lee KJ, Lee EH. 2018; With the greatest care, stromal interaction molecule (STIM) proteins verify what skeletal muscle is doing. BMB Rep. 51:378–387. DOI: 10.5483/BMBRep.2018.51.8.128. PMID: 29898810. PMCID: PMC6130827. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85055079049&origin=inward.4. Peake JM, Neubauer O, Della Gatta PA, Nosaka K. 2017; Muscle damage and inflammation during recovery from exercise. J Appl Physiol (1985). 122:559–570. DOI: 10.1152/japplphysiol.00971.2016. PMID: 28035017. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85014792249&origin=inward.5. Delos D, Maak TG, Rodeo SA. 2013; Muscle injuries in athletes: enhancing recovery through scientific understanding and novel therapies. Sports Health. 5:346–352. DOI: 10.1177/1941738113480934. PMID: 24459552. PMCID: PMC3899907. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84879182162&origin=inward.6. Thooyamani AS, Mukhopadhyay A. 2021; PDGFRα mediated survival of myofibroblasts inhibit satellite cell proliferation during aberrant regeneration of lacerated skeletal muscle. Sci Rep. 11:63. DOI: 10.1038/s41598-020-79771-4. PMID: 33420132. PMCID: PMC7794387. PMID: eb684f04a9c94611bd1374ec7155475d. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85098994697&origin=inward.7. Ribeiro AF Jr, Souza LS, Almeida CF, Ishiba R, Fernandes SA, Guerrieri DA, Santos ALF, Onofre-Oliveira PCG, Vainzof M. 2019; Muscle satellite cells and impaired late stage regeneration in different murine models for muscular dystrophies. Sci Rep. 9:11842. DOI: 10.1038/s41598-019-48156-7. PMID: 31413358. PMCID: PMC6694188. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85070946308&origin=inward.8. Verma M, Asakura Y, Murakonda BSR, Pengo T, Latroche C, Chazaud B, McLoon LK, Asakura A. 2018; Muscle satellite cell cross-talk with a vascular niche maintains quiescence via VEGF and Notch signaling. Cell Stem Cell. 23:530–543.e9. DOI: 10.1016/j.stem.2018.09.007. PMID: 30290177. PMCID: PMC6178221. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85053859628&origin=inward.9. Jeong JY, Kim JM, Rajesh RV, Suresh S, Jang GW, Lee KT, Kim TH, Park M, Jeong HJ, Kim KW, Cho YM, Lee HJ. 2013; Comparison of gene expression levels of porcine satellite cells from postnatal muscle tissue during differenti-ation. Reprod Dev Biol. 37:219–224. DOI: 10.12749/RDB.2013.37.4.219.10. Baghdadi MB, Tajbakhsh S. 2018; Regulation and phylogeny of skeletal muscle regeneration. Dev Biol. 433:200–209. DOI: 10.1016/j.ydbio.2017.07.026. PMID: 28811217. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85029700470&origin=inward.11. Forcina L, Cosentino M, Musarò A. 2020; Mechanisms regulating muscle regeneration: insights into the interrelated and time-dependent phases of tissue healing. Cells. 9:1297. DOI: 10.3390/cells9051297. PMID: 32456017. PMCID: PMC7290814. PMID: 7bfbfd899636487584d16c4b6e2f627c. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85085538839&origin=inward.12. Larrick JW, Larrick JW, Mendelsohn AR. 2016; Reversal of aged muscle stem cell dysfunction. Rejuvenation Res. 19:423–429. DOI: 10.1089/rej.2016.1875. PMID: 27612523. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84992200362&origin=inward.13. Pini V, Morgan JE, Muntoni F, O'Neill HC. 2017; Genome editing and muscle stem cells as a therapeutic tool for muscular dystrophies. Curr Stem Cell Rep. 3:137–148. DOI: 10.1007/s40778-017-0076-6. PMID: 28616376. PMCID: PMC5445179. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85045943044&origin=inward.14. Zhu P, Wu F, Mosenson J, Zhang H, He TC, Wu WS. 2017; CRISPR/Cas9-mediated genome editing corrects dystrophin mutation in skeletal muscle stem cells in a mouse model of muscle dystrophy. Mol Ther Nucleic Acids. 7:31–41. DOI: 10.1016/j.omtn.2017.02.007. PMID: 28624206. PMCID: PMC5363682. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85020720585&origin=inward.15. Alway SE, Myers MJ, Mohamed JS. 2014; Regulation of satellite cell function in sarcopenia. Front Aging Neurosci. 6:246. DOI: 10.3389/fnagi.2014.00246. PMID: 25295003. PMCID: PMC4170136. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84907942844&origin=inward.16. Said RS, Mustafa AG, Asfour HA, Shaqoura EI. 2017; Myogenic satellite cells: biological milieu and possible clinical applications. Pak J Biol Sci. 20:1–11. DOI: 10.3923/pjbs.2017.1.11. PMID: 29023009. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85006356043&origin=inward.17. Post MJ, Levenberg S, Kaplan DL, Genovese N, Fu J, Bryant CJ, Negowetti N, Verzijden K, Moutsatsou P. 2020; Scientific, sustainability and regulatory challenges of cultured meat. Nat Food. 1:403–415. DOI: 10.1038/s43016-020-0112-z. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85088867952&origin=inward.18. Choi KH, Yoon JW, Kim M, Lee HJ, Jeong J, Ryu M, Jo C, Lee CK. 2021; Muscle stem cell isolation and in vitro culture for meat production: a methodological review. Compr Rev Food Sci Food Saf. 20:429–457. DOI: 10.1111/1541-4337.12661. PMID: 33443788. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85096673947&origin=inward.19. Stephens N, Di Silvio L, Dunsford I, Ellis M, Glencross A, Sexton A. 2018; Bringing cultured meat to market: technical, socio-political, and regulatory challenges in cellular agriculture. Trends Food Sci Technol. 78:155–166. DOI: 10.1016/j.tifs.2018.04.010. PMID: 30100674. PMCID: PMC6078906. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85048566593&origin=inward.20. Treich N. 2021; Cultured meat: promises and challenges. Environ Resour Econ (Dordr). doi: 10.1007/s10640-021-00551-3. [Epub ahead of print]. DOI: 10.1007/s10640-021-00551-3. PMID: 33758465. PMCID: PMC7977488. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85103064615&origin=inward.21. Srutee R, R S S, Uday S A. 2021; Clean meat: techniques for meat production and its upcoming challenges. Anim Biotechnol. doi: 10.1080/10495398.2021.1911810. [Epub ahead of print]. DOI: 10.1080/10495398.2021.1911810. PMID: 33947302. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85105328829&origin=inward.22. Yin H, Price F, Rudnicki MA. 2013; Satellite cells and the muscle stem cell niche. Physiol Rev. 93:23–67. DOI: 10.1152/physrev.00043.2011. PMID: 23303905. PMCID: PMC4073943. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84872281980&origin=inward.23. McKay BR, Nederveen JP, Fortino SA, Snijders T, Joanisse S, Kumbhare DA, Parise G. 2020; Brain-derived neurotrophic factor is associated with human muscle satellite cell differentiation in response to muscle-damaging exercise. Appl Physiol Nutr Metab. 45:581–590. DOI: 10.1139/apnm-2019-0501. PMID: 31661631. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85085904103&origin=inward.24. Shahini A, Vydiam K, Choudhury D, Rajabian N, Nguyen T, Lei P, Andreadis ST. 2018; Efficient and high yield isolation of myoblasts from skeletal muscle. Stem Cell Res. 30:122–129. DOI: 10.1016/j.scr.2018.05.017. PMID: 29879622. PMCID: PMC6090567. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85047855425&origin=inward.25. Yoshioka K, Kitajima Y, Okazaki N, Chiba K, Yonekura A, Ono Y. 2020; A modified pre-plating method for high-yield and high-purity muscle stem cell isolation from human/mouse skeletal muscle tissues. Front Cell Dev Biol. 8:793. DOI: 10.3389/fcell.2020.00793. PMID: 32903486. PMCID: PMC7438441. PMID: 76aca5e166e449acb21aac192da73336. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85089938198&origin=inward.26. Motohashi N, Asakura Y, Asakura A. 2014; Isolation, culture, and transplantation of muscle satellite cells. J Vis Exp. (86):50846. DOI: 10.3791/50846. PMID: 24747722. PMCID: PMC4131689. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84898746462&origin=inward.27. Benedetti A, Cera G, De Meo D, Villani C, Bouche M, Lozanoska-Ochser B. 2021; A novel approach for the isolation and long-term expansion of pure satellite cells based on ice-cold treatment. Skelet Muscle. 11:7. DOI: 10.1186/s13395-021-00261-w. PMID: 33731194. PMCID: PMC7968259. PMID: 3a65cd8f1149438a87f3bea3700016a6. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85102718477&origin=inward.28. Gromova A, Tierney MT, Sacco A. 2015; FACS-based satellite cell isolation from mouse hind limb muscles. Bio Protoc. 5:e1558. DOI: 10.21769/BioProtoc.1558. PMID: 27668269. PMCID: PMC5034768.29. Maesner CC, Almada AE, Wagers AJ. 2016; Established cell surface markers efficiently isolate highly overlapping populations of skeletal muscle satellite cells by fluorescence-activated cell sorting. Skelet Muscle. 6:35. DOI: 10.1186/s13395-016-0106-6. PMID: 27826411. PMCID: PMC5100091. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84999158343&origin=inward.30. Syverud BC, Lee JD, VanDusen KW, Larkin LM. 2014; Isolation and purification of satellite cells for skeletal muscle tissue engineering. J Regen Med. 3:117. DOI: 10.4172/2325-9620.1000117. PMID: 26413555. PMCID: PMC4582791.31. Singh A, Benjakul S. 2018; Proteolysis and its control using protease inhibitors in fish and fish products: a review. Compr Rev Food Sci Food Saf. 17:496–509. DOI: 10.1111/1541-4337.12337. PMID: 33350077. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85042024856&origin=inward.32. Miersch C, Stange K, Röntgen M. 2018; Effects of trypsinization and of a combined trypsin, collagenase, and DNase digestion on liberation and in vitro function of satellite cells isolated from juvenile porcine muscles. In Vitro Cell Dev Biol Anim. 54:406–412. DOI: 10.1007/s11626-018-0263-5. PMID: 29785535. PMCID: PMC5997727. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85047194017&origin=inward.33. Brown NK, Meade JR, Wang J, Marino SR. 2017; Reanalysis of the role of pronase treatment of B cells in the flow cytometric crossmatch assay: Fc receptor is not the primary target. Hum Immunol. 78:704–709. DOI: 10.1016/j.humimm.2017.10.002. PMID: 28987959. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85030788393&origin=inward.34. Bentzinger CF, Wang YX, von Maltzahn J, Soleimani VD, Yin H, Rudnicki MA. 2013; Fibronectin regulates Wnt7a signaling and satellite cell expansion. Cell Stem Cell. 12:75–87. DOI: 10.1016/j.stem.2012.09.015. PMID: 23290138. PMCID: PMC3539137. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84872009392&origin=inward.35. Park MH, Kim MS, Yun JI, Choi JH, Lee E, Lee ST. 2018; Integrin heterodimers expressed on the surface of porcine spermatogonial stem cells. DNA Cell Biol. 37:253–263. DOI: 10.1089/dna.2017.4035. PMID: 29369695. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85045425624&origin=inward.36. Kim SJ, Kim MS, Park HJ, Lee H, Yun JI, Lim HW, Lee ST. 2020; Screening of integrins localized on the surface of human epidermal melanocytes. In Vitro Cell Dev Biol Anim. 56:435–443. DOI: 10.1007/s11626-020-00471-4. PMID: 32572848. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85086778940&origin=inward.37. Relaix F, Zammit PS. 2012; Satellite cells are essential for skeletal muscle regeneration: the cell on the edge returns centre stage. Development. 139:2845–2856. DOI: 10.1242/dev.069088. PMID: 22833472. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84864317860&origin=inward.38. Butchart LC, Fox A, Shavlakadze T, Grounds MD. 2016; The long and short of non-coding RNAs during post-natal growth and differentiation of skeletal muscles: focus on lncRNA and miRNAs. Differentiation. 92:237–248. DOI: 10.1016/j.diff.2016.05.003. PMID: 27292314. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84998694304&origin=inward.39. White RB, Biérinx AS, Gnocchi VF, Zammit PS. 2010; Dynamics of muscle fibre growth during postnatal mouse develop-ment. BMC Dev Biol. 10:21. DOI: 10.1186/1471-213X-10-21. PMID: 20175910. PMCID: PMC2836990. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=77649309210&origin=inward.40. Gharaibeh B, Lu A, Tebbets J, Zheng B, Feduska J, Crisan M, Péault B, Cummins J, Huard J. 2008; Isolation of a slowly adhering cell fraction containing stem cells from murine skeletal muscle by the preplate technique. Nat Protoc. 3:1501–1509. DOI: 10.1038/nprot.2008.142. PMID: 18772878. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=51649123800&origin=inward.41. Lavasani M, Lu A, Thompson SD, Robbins PD, Huard J, Niedernhofer LJ. 2013; Isolation of muscle-derived stem/progenitor cells based on adhesion characteristics to collagen-coated surfaces. Methods Mol Biol. 976:53–65. DOI: 10.1007/978-1-62703-317-6_5. PMID: 23400434. PMCID: PMC3731996. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84880576445&origin=inward.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recent Advances in Skeletal Muscle Stem Cells for Duchenne Muscular Dystrophy Treatment
- Exercise/Resistance Training and Muscle Stem Cells
- Possible Local Stem Cells Activation by Microcurrent Application in Experimentally Injured Soleus Muscle
- Bex1 Participates in Muscle Regeneration by Regulating Myogenic Satellite Cell Differentiation
- Isolation and Characterization of Human Muscle Cells