Lab Anim Res.
2010 Jun;26(2):145-151.
Bex1 Participates in Muscle Regeneration by Regulating Myogenic Satellite Cell Differentiation
- Affiliations
-
- 1Department of Anatomy and Neurobiology, School of Medicine, University of Maryland, Baltimore, USA. jkoo001@umaryland.edu
Abstract
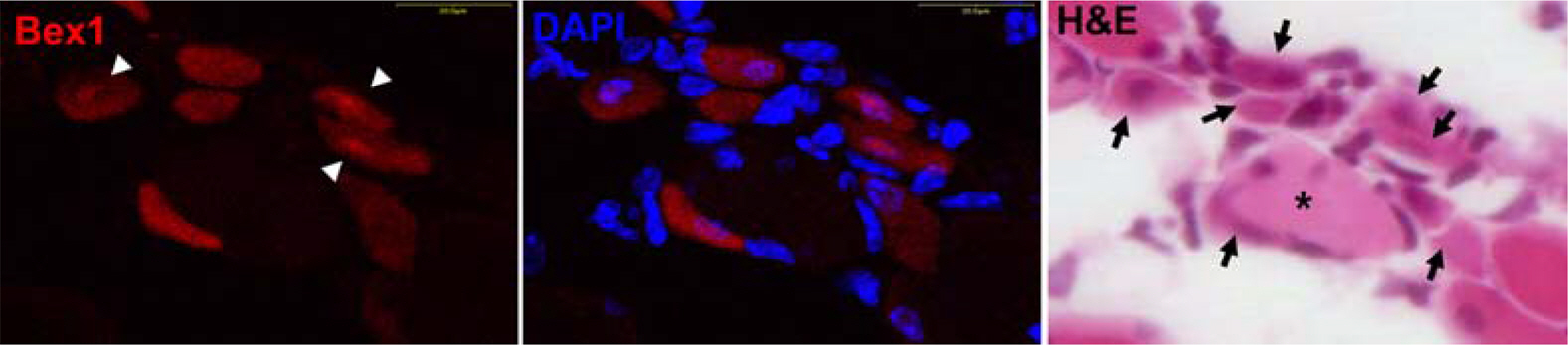
- Bex1 protein is upregulated in regenerating muscle and interacts with calmodulin, a Ca2+-binding protein involved in cell cycle regulation. Following cardiotoxin-induced injury the regenerating muscle of Bex1 knock-out mice exhibits prolonged cell proliferation and delayed cell differentiation compared to wild-type mice. To gain insight into this process, we compared the regenerating myogenic morphologies of Bex1 knock-out and wild-type mice at several time points. Bex1-positive cells were identified by double immunofluorescence staining. These studies demonstrated that a population of cells that are Bex1-positive after injury are c-Met/basal lamina-positive and Mac-1-negative indicating that they are derived from at least a subset of myogenic progenitor/satellite cells but not invading immune cells. In addition, in regenerating muscle, Bex1 co-localizes with calmodulin in the cytoplasm of the late myoblast or early myotube stage of myogenesis. These results suggest that Bex1 participates in muscle regeneration through the regulation of satellite cell proliferation and differentiation by its interaction with calmodulin. Current studies of Bex1 may provide a new molecular tool for the identification of activated satellite cell and open the way to new or improved therapeutic regimens against progressive muscular atrophy.
Keyword
MeSH Terms
Figure
Reference
-
Alvarez E.., Zhou W.., Witta S.E.., Freed C.R.2005. Characterization of the Bex gene family in humans, mice, and rats. Gene. 357(1):18–28.
ArticleBehrens M.., Margolis J.W.., Margolis F.L.2003. Identification of members of the Bex gene family as olfactory marker protein (OMP) binding partners. J. Neurochem. 86(5):1289–1296.
ArticleBurghes A.H.., Logan C.., Hu X.., Belfall B.., Worton R.G.., Ray P.N.1987. A cDNA clone from the Duchenne/Becker muscular dystrophy gene. Nature. 328(6129):434–437.
ArticleCharge S.B.., Rudnicki M.A.2004. Cellular and molecular regulation of muscle regeneration. Physiol. Rev. 84(1):209–238.Cornelison D.D.., Wold B.J.1997. Single-cell analysis of regulatory gene expression in quiescent and activated mouse skeletal muscle satellite cells. Dev. Biol. 191(2):270–283.
ArticleCornelison D.D.., Filla M.S.., Stanley H.M.., Rapraeger A.C.., Olwin B.B.2001. Syndecan-3 and syndecan-4 specifically mark skeletal muscle satellite cells and are implicated in satellite cell maintenance and muscle regeneration. Dev. Biol. 239(1):79–94.
ArticleCossu G.., Mavilio F.2000. Myogenic stem cells for the therapy of primary myopathies: wishful thinking or therapeutic perspective? J. Clin. Invest. 105(12):1669–1674.
ArticleDhawan J.., Rando T.A.2005. Stem cells in postnatal myogenesis: molecular mechanisms of satellite cell quiescence, activation and replenishment. Trends Cell Biol. 15(12):666–673.
ArticleFloss T.., Arnold H.H.., Braun T.1997. A role for FGF-6 in skeletal muscle regeneration. Genes Dev. 11(16):2040–2051.Garry D.J.., Yang Q.., Bassel-Duby R.., Williams R.S.1997. Persistent expression of MNF identifies myogenic stem cells in postnatal muscles. Dev. Biol. 188(2):280–294.
ArticleGarry D.J.., Meeson A.., Elterman J.., Zhao Y.., Yang P.., Bassel-Duby R.., Williams R.S.2000. Myogenic stem cell function is impaired in mice lacking the forkhead/winged helix protein MNF. Proc. Natl. Acad. Sci. USA. 97(10):5416–5421.
ArticleGrady R.M.., Teng H.., Nichol M.C.., Cunningham J.C.., Wilkinson R.S.., Sanes J.R.1997. Skeletal and cardiac myopathies in mice lacking utrophin and dystrophin: a model for Duchenne muscular dystrophy. Cell. 90(4):729–738.
ArticleGoetsch S.C.., Hawke T.J.., Gallardo T.D.., Richardson J.A.., Garry D.J.2003. Transcriptional profiling and regulation of the extracellular matrix during muscle regeneration. Physiol. Genomics. 14(3):261–271.
ArticleHawke T.J.., Garry D.J.2001. Myogenic satellite cells: physiology to molecular biology. J. Appl. Physiol. 91(2):534–551.
ArticleHeslop L.., Morgan J.E.., Partridge T.A.2000. Evidence for a myogenic stem cell that is exhausted in dystrophic muscle. J. Cell Sci. 113(12):2299–2308.
ArticleKoo J.H.., Saraswati M.., Margolis F.L.2005. Immunolocalization of Bex protein in the mouse brain and olfactory system. J. Comp. Neurol. 487(1):1–14.
ArticleKoo J.H.., Smiley M.A.., Lovering R.M.., Margolis F.L.2007. Bex1 knock out mice show altered skeletal muscle regeneration. Biochem. Biophys. Res. Commun. 363(2):405–410.
ArticleMorgan J.E.., Partridge T.A.2003. Muscle satellite cells. Int. J. Biochem. Cell Biol. 35(8):1151–1156.
ArticleOrimo S.., Hiyamuta E.., Arahata K.., Sugita H.1991. Analysis of inflammatory cells and complement C3 in bupivacaine-induced myonecrosis. Muscle Nerve. 14(6):515–520.
ArticleSchmalbruch H.., Lewis D.M.2000. Dynamics of nuclei of muscle fibers and connective tissue cells in normal and denervated rat muscles. Muscle Nerve. 23(4):617–626.
ArticleTidball J.G.., Berchenko E.., Frenette J.1999. Macrophage invasion does not contribute to muscle membrane injury during inflammation. J. Leukoc. Biol. 65(4):492–498.
ArticleVilar M.., Murillo-Carretero M.., Mira H.., Magnusson K.., Besset V.., Ibáñez C.F.2006. Bex1, a novel interactor of the p75 neurotrophin receptor, links neurotrophin signaling to the cell cycle. EMBO J. 25(6):1219–1230.
ArticleYan Z.., Choi S.., Liu X.., Zhang M.., Schageman J.J.., Lee S.Y.., Hart R.., Lin L.., Thurmond F.A.., Williams R.S.2003. Highly coordinated gene regulation in mouse skeletal muscle regeneration. J. Biol. Chem. 278(10):8826–8836.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recent Advances in Skeletal Muscle Stem Cells for Duchenne Muscular Dystrophy Treatment
- MBP-FGF2-Immobilized Matrix Maintains Self-Renewal and Myogenic Differentiation Potential of Skeletal Muscle Stem Cells
- Skeletal myogenic differentiation of human periodontal ligament stromal cells isolated from orthodontically extracted premolars
- Saturated fatty acid-inducible miR-103-3p impairs the myogenic differentiation of progenitor cells by enhancing cell proliferation through Twinfilin-1/F-actin/YAP1 axis
- From Barbells to Brawns: The Physiology of Resistance Exercise and Skeletal Muscle Growth