Int J Stem Cells.
2022 Aug;15(3):270-282. 10.15283/ijsc21088.
Abnormal Development of Neural Stem Cell Niche in the Dentate Gyrus of Menkes Disease
- Affiliations
-
- 1Cell Therapy Research Center, GC Cell, Yongin, Korea
- 2Department of Neurology, Dongguk University Ilsan Hospital, Goyang, Korea
- 3Department of Pediatrics, Seoul National University Children’s Hospital, Seoul National University College of Medicine, Seoul, Korea
- KMID: 2532401
- DOI: http://doi.org/10.15283/ijsc21088
Abstract
- Background and Objectives
Menkes disease (MNK) is a rare X-linked recessive disease, caused by mutations in the copper transporting ATP7A gene that is required for copper homeostasis. MNK patients experience various clinical symptoms including neurological defects that are closely related to the prognosis of MNK patients. Neural stem cells (NSCs) in the hippocampal dentate gyrus (DG) produce new neurons throughout life, and defects in DG neurogenesis are often correlated with cognitive and behavioral problems. However, neurodevelopmental defects in the DG during postnatal period in MNK have not been understood yet.
Methods and Results
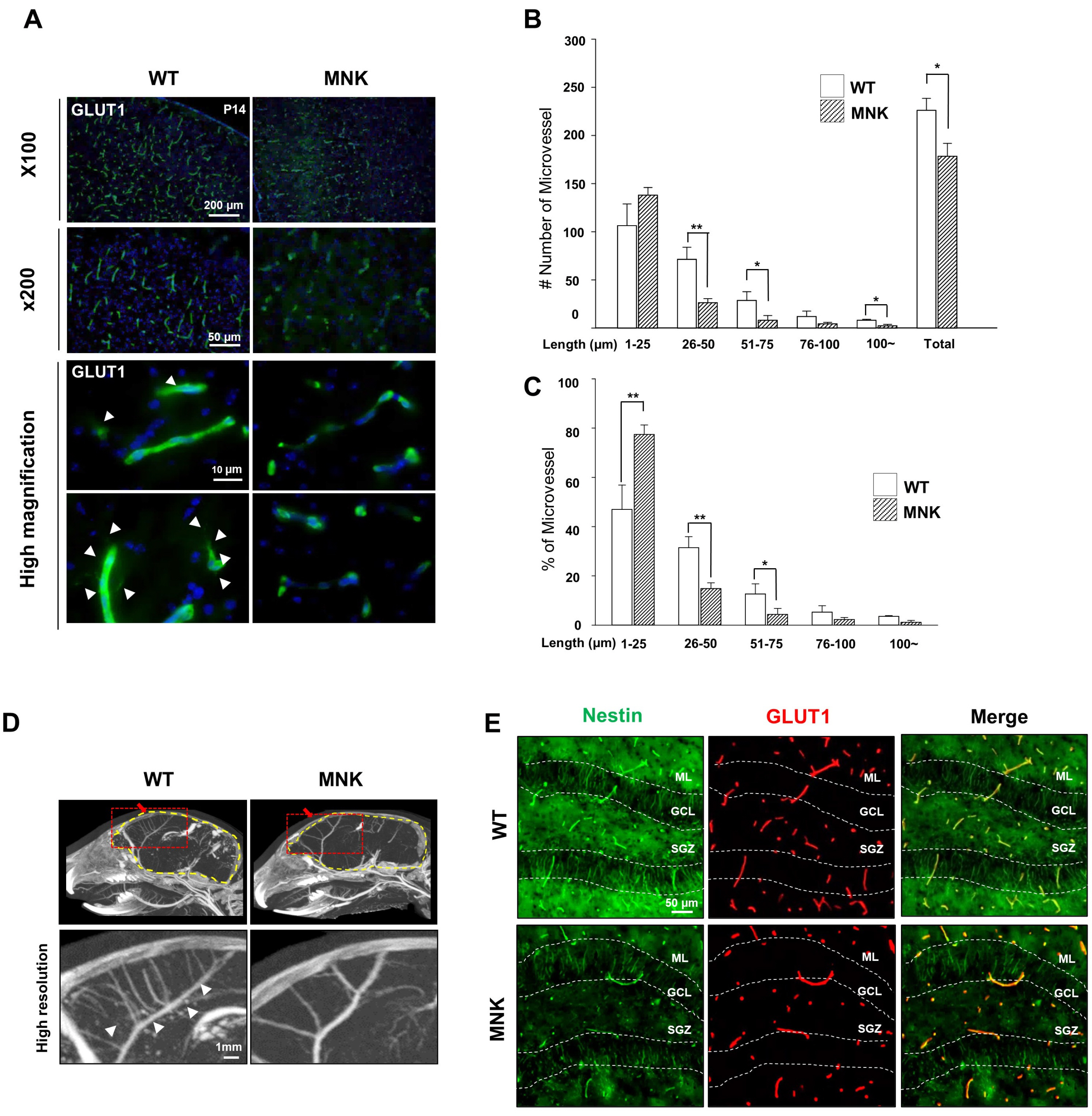
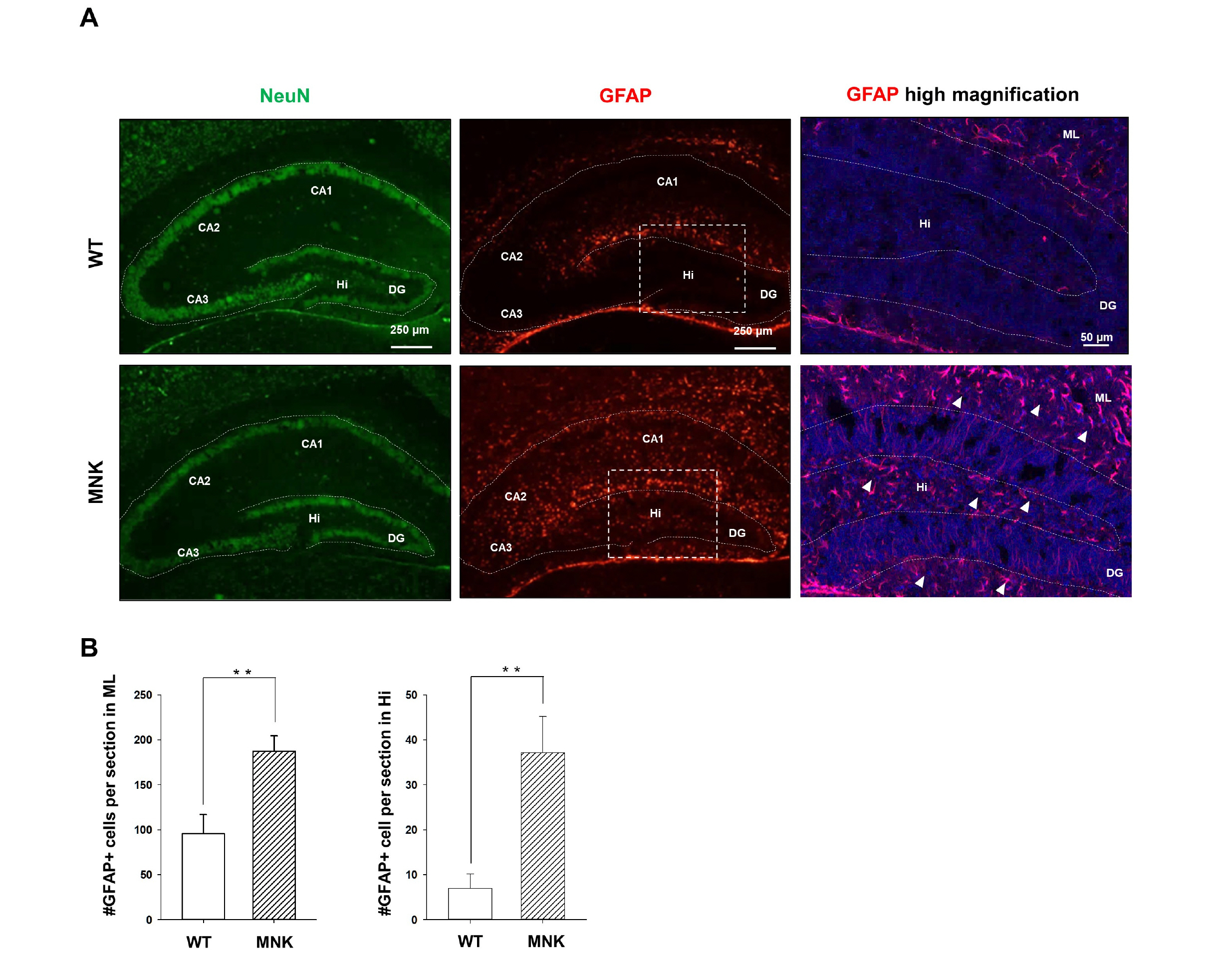
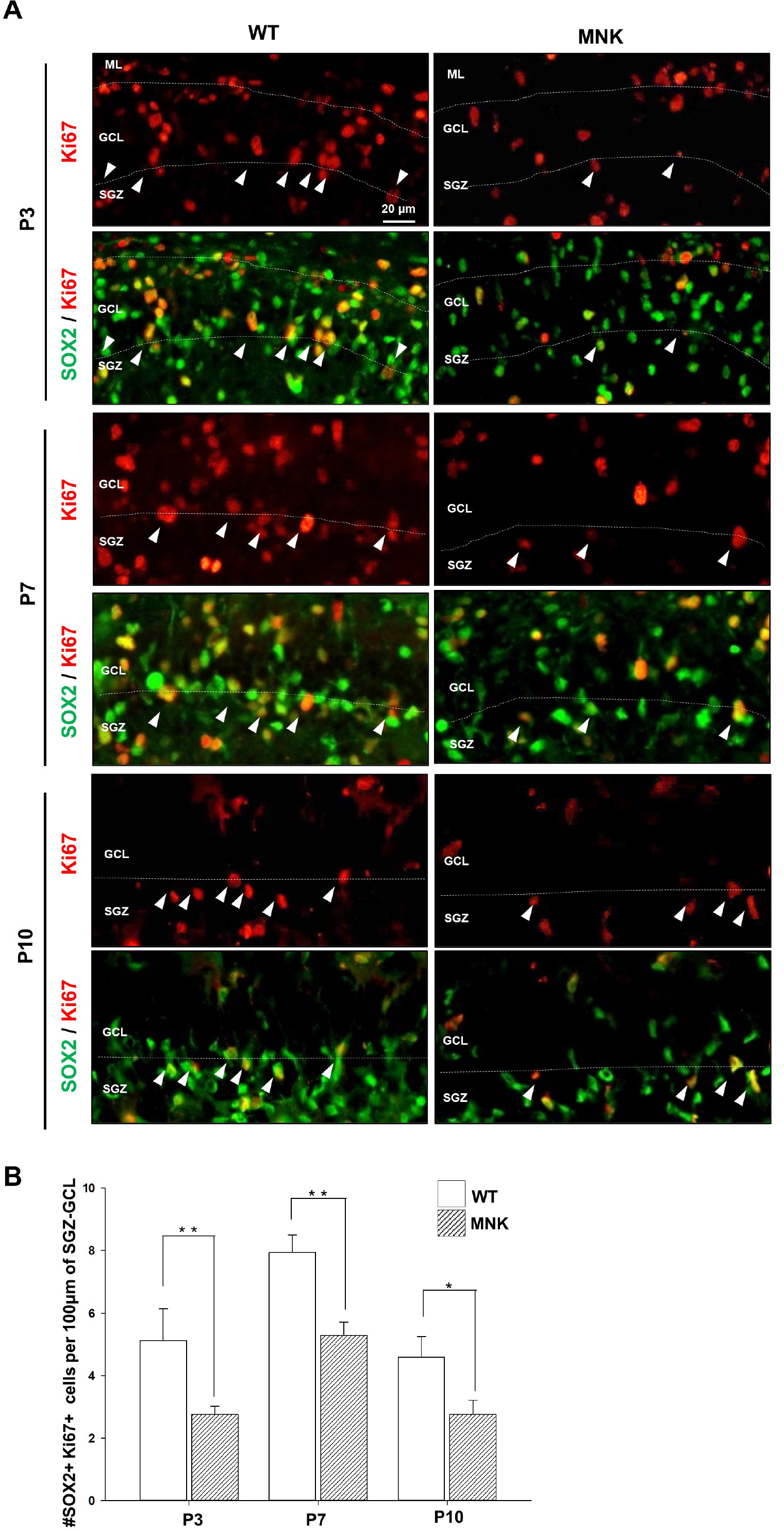
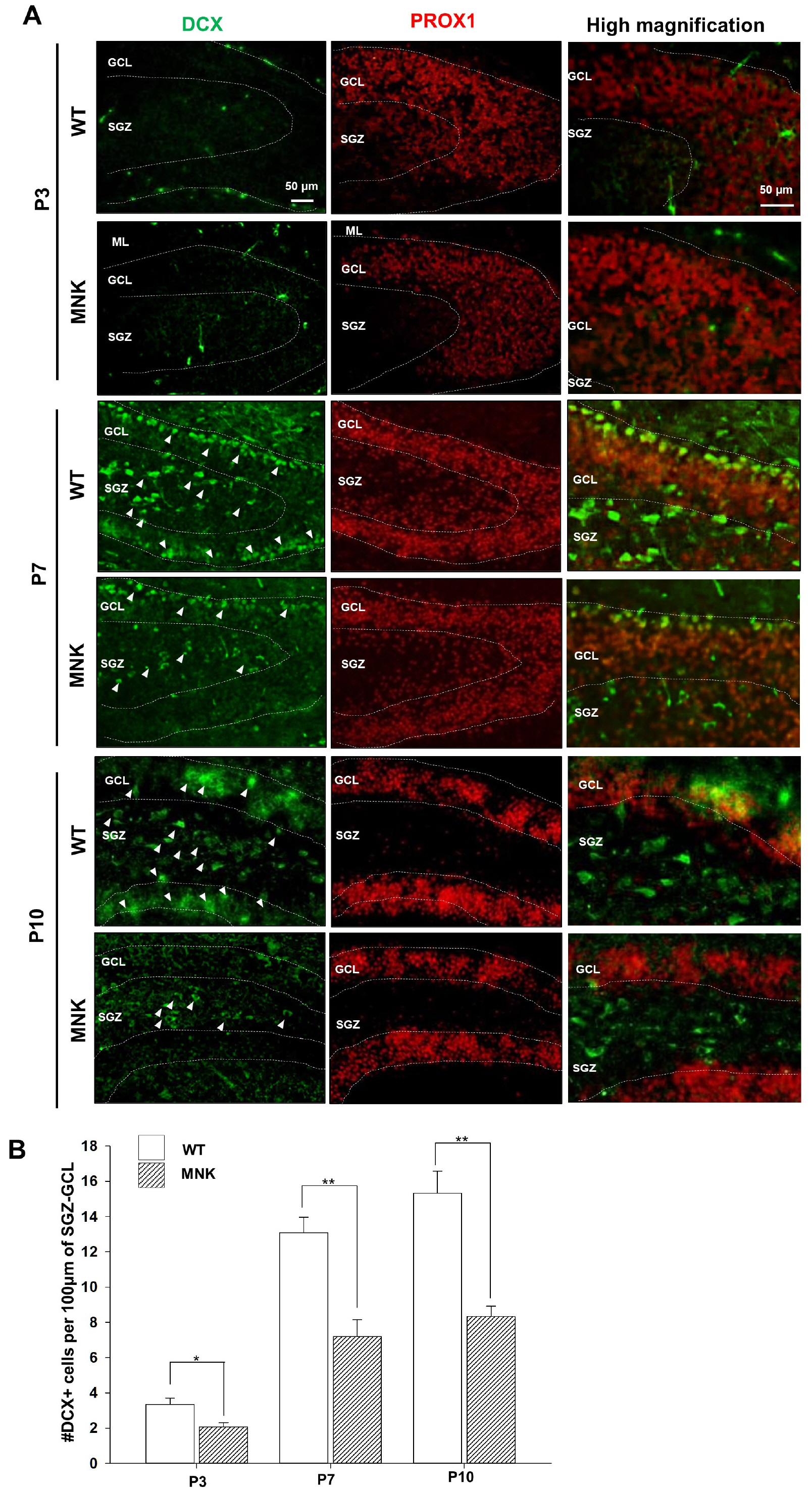
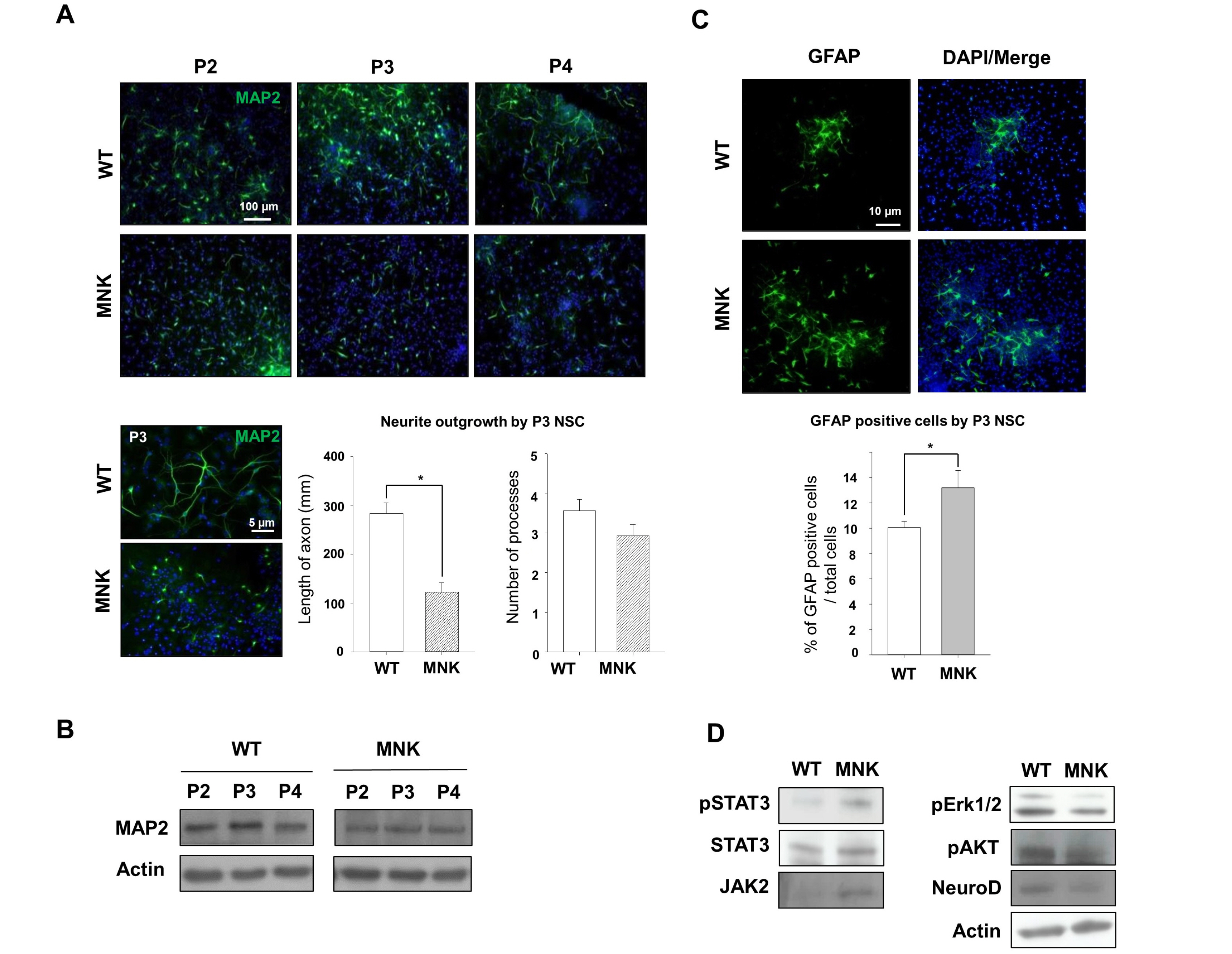
Mottled-brindled (Mo Br/y ) mice (MNK mice) and littermate controls were used in this study. In vivo microCT imaging and immunohistochemistry results demonstrate that blood vasculatures in hippocampus are abnormally decreased in MNK mice. Furthermore, postnatal establishment of NSC population and their neurogenesis are severely compromised in the DG of MNK mice. In addition, in vitro analyses using hippocampal neurosphere culture followed by immunocytochemistry and immunoblotting suggest that neurogenesis from MNK NSCs is also significantly compromised, corresponding to defective neurogenic gene expression in MNK derived neurons.
Conclusions
Our study is the first reports demonstrating that improper expansion of the postnatal NSC population followed by significant reduction of neurogenesis may contribute to neurodevelopmental symptoms in MNK. In conclusion, our results provide new insight into early neurodevelopmental defects in MNK and emphasize the needs for early diagnosis and new therapeutic strategies in the postnatal central nerve system damage of MNK patients.
Figure
Reference
-
References
1. Vulpe C, Levinson B, Whitney S, Packman S, Gitschier J. 1993; Isolation of a candidate gene for Menkes disease and evidence that it encodes a copper-transporting ATPase. Nat Genet. 7–13. Erratum in: Nat Genet 1993;3:273. DOI: 10.1038/ng0193-7. PMID: 8490659. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0027446365&origin=inward.2. Mercer JF, Livingston J, Hall B, Paynter JA, Begy C, Chandrasekharappa S, Lockhart P, Grimes A, Bhave M, Siemieniak D, Glover TW. 1993; Isolation of a partial candidate gene for Menkes disease by positional cloning. Nat Genet. 3:20–25. DOI: 10.1038/ng0193-20. PMID: 8490647. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0027475976&origin=inward.3. Menkes JH, Alter M, Steigleder GK, Weakley DR, Sung JH. 1962; A sex-linked recessive disorder with retardation of growth, peculiar hair, and focal cerebral and cerebellar degeneration. Pediatrics. 29:764–779. PMID: 14472668. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=78651124591&origin=inward.4. Kaler SG. 2011; ATP7A-related copper transport diseases-emerging concepts and future trends. Nat Rev Neurol. 7:15–29. DOI: 10.1038/nrneurol.2010.180. PMID: 21221114. PMCID: PMC4214867. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=78651355486&origin=inward.5. Paynter JA, Grimes A, Lockhart P, Mercer JF. 1994; Expression of the Menkes gene homologue in mouse tissues lack of effect of copper on the mRNA levels. FEBS Lett. 351:186–190. DOI: 10.1016/0014-5793(94)00868-X. PMID: 8082762. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0027968802&origin=inward.6. Madsen E, Gitlin JD. 2007; Copper and iron disorders of the brain. Annu Rev Neurosci. 30:317–337. DOI: 10.1146/annurev.neuro.30.051606.094232. PMID: 17367269. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=34547782810&origin=inward.7. Kodama H, Fujisawa C, Bhadhprasit W. 2012; Inherited copper transport disorders: biochemical mechanisms, diagnosis, and treatment. Curr Drug Metab. 13:237–250. DOI: 10.2174/138920012799320455. PMID: 21838703. PMCID: PMC3290776. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84860505334&origin=inward.8. Qian Y, Tiffany-Castiglioni E, Welsh J, Harris ED. 1998; Copper efflux from murine microvascular cells requires expression of the menkes disease Cu-ATPase. J Nutr. 128:1276–1282. DOI: 10.1093/jn/128.8.1276. PMID: 9687544. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0031829282&origin=inward.9. Kaler SG, DiStasio AT. Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Gripp KW, Mirzaa GM, Amemiya A, editors. 1993. ATP7A-related copper transport disorders. GeneReviews®. University of Washington;Seattle: PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0027968802&origin=inward.10. Donsante A, Yi L, Zerfas PM, Brinster LR, Sullivan P, Goldstein DS, Prohaska J, Centeno JA, Rushing E, Kaler SG. 2011; ATP7A gene addition to the choroid plexus results in long-term rescue of the lethal copper transport defect in a Menkes disease mouse model. Mol Ther. 19:2114–2123. DOI: 10.1038/mt.2011.143. PMID: 21878905. PMCID: PMC3242653. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=82955232408&origin=inward.11. Deng W, Aimone JB, Gage FH. 2010; New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 11:339–350. DOI: 10.1038/nrn2822. PMID: 20354534. PMCID: PMC2886712. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=77951498581&origin=inward.12. Ming GL, Song H. 2011; Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neu-ron. 70:687–702. DOI: 10.1016/j.neuron.2011.05.001. PMID: 21609825. PMCID: PMC3106107. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=79956209852&origin=inward.13. Jacob FD, Habas PA, Kim K, Corbett-Detig J, Xu D, Studholme C, Glenn OA. 2011; Fetal hippocampal development: analysis by magnetic resonance imaging volumetry. Pediatr Res. 69(5 Pt 1):425–429. DOI: 10.1203/PDR.0b013e318211dd7f. PMID: 21270675. PMCID: PMC3132078. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=79954588964&origin=inward.14. Yu DX, Marchetto MC, Gage FH. 2014; How to make a hippocampal dentate gyrus granule neuron. Development. 141:2366–2375. DOI: 10.1242/dev.096776. PMID: 24917496. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84902189439&origin=inward.15. Wegiel J, Kuchna I, Nowicki K, Imaki H, Wegiel J, Marchi E, Ma SY, Chauhan A, Chauhan V, Bobrowicz TW, de Leon M, Louis LA, Cohen IL, London E, Brown WT, Wisniewski T. 2010; The neuropathology of autism: defects of neurogenesis and neuronal migration, and dysplastic changes. Acta Neuropathol. 119:755–770. DOI: 10.1007/s00401-010-0655-4. PMID: 20198484. PMCID: PMC2869041. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=77953028667&origin=inward.16. Mann JR, Camakaris J, Danks DM. 1979; Copper metabolism in mottled mouse mutants: distribution of 64Cu in brindled (Mobr) mice. Biochem J. 180:613–619. DOI: 10.1042/bj1800613. PMID: 573619. PMCID: PMC1161101. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0018791656&origin=inward.17. Emanuele P, Goodman ZD. 1998; A simple and rapid stain for copper in liver tissue. Ann Diagn Pathol. 2:125–126. DOI: 10.1016/S1092-9134(98)80049-X. PMID: 9845729. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0032056045&origin=inward.18. Weiss S, Dunne C, Hewson J, Wohl C, Wheatley M, Peterson AC, Reynolds BA. 1996; Multipotent CNS stem cells are present in the adult mammalian spinal cord and ventricular neuroaxis. J Neurosci. 16:7599–7609. DOI: 10.1523/JNEUROSCI.16-23-07599.1996. PMID: 8922416. PMCID: PMC6579089. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0029860591&origin=inward.19. Ding Y, Zhang Z, Ma J, Xia H, Wang Y, Liu Y, Ma Q, Sun T, Liu J. 2016; Directed differentiation of postnatal hippocampal neural stem cells generates nuclear receptor related-1 protein- and tyrosine hydroxylase‑expressing cells. Mol Med Rep. 14:1993–1999. DOI: 10.3892/mmr.2016.5489. PMID: 27432537. PMCID: PMC4991738. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84989201357&origin=inward.20. Wachs FP, Couillard-Despres S, Engelhardt M, Wilhelm D, Ploetz S, Vroemen M, Kaesbauer J, Uyanik G, Klucken J, Karl C, Tebbing J, Svendsen C, Weidner N, Kuhn HG, Winkler J, Aigner L. 2003; High efficacy of clonal growth and expansion of adult neural stem cells. Lab Invest. 83:949–962. DOI: 10.1097/01.LAB.0000075556.74231.A5. PMID: 12861035. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0041364517&origin=inward.21. Doetsch F, Caillé I, Lim DA, García-Verdugo JM, Alvarez-Buylla A. 1999; Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 97:703–716. DOI: 10.1016/S0092-8674(00)80783-7. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0033040497&origin=inward.22. Kodama H. 1993; Recent developments in Menkes disease. J Inherit Metab Dis. 16:791–799. DOI: 10.1007/BF00711911. PMID: 8412022. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0027236562&origin=inward.23. Tiffany-Castiglion E, Qian Y. 2001; Astroglia as metal depots: molecular mechanisms for metal accumulation, storage and release. Neurotoxicology. 22:577–592. DOI: 10.1016/S0161-813X(01)00050-X. PMID: 11770879. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0035211681&origin=inward.24. Gonzalez-Perez O, Quiñones-Hinojosa A. 2012; Astrocytes as neural stem cells in the adult brain. J Stem Cells. 7:181–188. DOI: 10.1155/2012/378356. PMID: 23213339. PMCID: PMC3504454. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84870185940&origin=inward.25. Suzuki J, Takaku A. 1969; Cerebrovascular "moyamoya" disease. Disease showing abnormal net-like vessels in base of brain. Arch Neurol. 20:288–299. DOI: 10.1001/archneur.1969.00480090076012. PMID: 5775283. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0014477593&origin=inward.26. Palmer TD, Willhoite AR, Gage FH. 2000; Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 425:479–494. DOI: 10.1002/1096-9861(20001002)425:4<479::AID-CNE2>3.0.CO;2-3. PMID: 10975875.27. Patrício P, Mateus-Pinheiro A, Sousa N, Pinto L. 2013; Re-cycling paradigms: cell cycle regulation in adult hippocampal neurogenesis and implications for depression. Mol Neuro-biol. 48:84–96. DOI: 10.1007/s12035-013-8422-x. PMID: 23471746. PMCID: PMC3718990. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84880924582&origin=inward.28. Nicola Z, Fabel K, Kempermann G. 2015; Development of the adult neurogenic niche in the hippocampus of mice. Front Neuroanat. 9:53. DOI: 10.3389/fnana.2015.00053. PMID: 25999820. PMCID: PMC4423450. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84930615910&origin=inward.29. Negishi T, Ishii Y, Kyuwa S, Kuroda Y, Yoshikawa Y. 2003; Primary culture of cortical neurons, type-1 astrocytes, and microglial cells from cynomolgus monkey (Macaca fascicularis) fetuses. J Neurosci Methods. 131:133–140. DOI: 10.1016/j.jneumeth.2003.08.006. PMID: 14659833. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0344945396&origin=inward.30. Rajan P, McKay RD. 1998; Multiple routes to astrocytic differentiation in the CNS. J Neurosci. 18:3620–3629. DOI: 10.1523/JNEUROSCI.18-10-03620.1998. PMID: 9570793. PMCID: PMC6793143. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0032525155&origin=inward.31. Rhim JH, Luo X, Gao D, Xu X, Zhou T, Li F, Wang P, Wong ST, Xia X. 2016; Cell type-dependent Erk-Akt pathway crosstalk regulates the proliferation of fetal neural progenitor cells. Sci Rep. 6:26547. DOI: 10.1038/srep26547. PMID: 27211495. PMCID: PMC4876380. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84970005266&origin=inward.32. Peltier J, O'Neill A, Schaffer DV. 2007; PI3K/Akt and CREB regulate adult neural hippocampal progenitor proliferation and differentiation. Dev Neurobiol. 67:1348–1361. DOI: 10.1002/dneu.20506. PMID: 17638387. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=34547793039&origin=inward.33. Shioda N, Han F, Fukunaga K. 2009; Role of Akt and ERK signaling in the neurogenesis following brain ischemia. Int Rev Neurobiol. 85:375–387. DOI: 10.1016/S0074-7742(09)85026-5. PMID: 19607982. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=67650079547&origin=inward.34. Cosimo QC, Daniela L, Elsa B, Carlo DV, Giuseppe F. 2011; Kinky hair, kinky vessels, and bladder diverticula in Menkes disease. J Neuroimaging. 21:e114–e116. DOI: 10.1111/j.1552-6569.2010.00476.x. PMID: 20412396. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=79953040569&origin=inward.35. Kishimoto T, Fukuzawa Y, Abe M, Hashimoto M, Ohno M, Tada M. 1992; Injury to cultured human vascular endothelial cells by copper (CuSO4). Nihon Eiseigaku Zasshi. 47:965–970. DOI: 10.1265/jjh.47.965. PMID: 1287265. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0027090048&origin=inward.36. Goldberg JS, Hirschi KK. 2009; Diverse roles of the vasculature within the neural stem cell niche. Regen Med. 4:879–897. DOI: 10.2217/rme.09.61. PMID: 19903006. PMCID: PMC2836203. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=75649144081&origin=inward.37. Shen Q, Temple S. 2009; Fine control: microRNA regulation of adult neurogenesis. Nat Neurosci. 12:369–370. DOI: 10.1038/nn0409-369. PMID: 19322237. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=63649088898&origin=inward.38. Suh JH, Kim D, Kim H, Helfman DM, Choi JH, Lee BH, Yoo HW, Han YM. 2014; Modeling of Menkes disease via human induced pluripotent stem cells. Biochem Biophys Res Co-mmun. 444:311–318. DOI: 10.1016/j.bbrc.2014.01.038. PMID: 24468087. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84894144906&origin=inward.39. Watanabe M, Tezuka M. 2006; Copper is required for retinoic acid receptor-dependent transcription and neuronal differentiation in P19 embryonal carcinoma cells. J Health Sci. 52:540–548. DOI: 10.1248/jhs.52.540. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=33749435734&origin=inward.40. Birkaya B, Aletta JM. 2005; NGF promotes copper accumulation required for optimum neurite outgrowth and protein methylation. J Neurobiol. 63:49–61. DOI: 10.1002/neu.20114. PMID: 15627265. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=14844337444&origin=inward.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Adult Neurogenesis in the Central and Peripheral Nervous Systems
- Endogenous Neurogenesis in Postnatal Brain
- Polarized and Stage-Dependent Distribution of Immunoreactivity for Novel PDZ-Binding Protein Preso1 in Adult Neurogenic Regions
- Toll-like receptor 2 promotes neurogenesis from the dentate gyrus after photothrombotic cerebral ischemia in mice
- Comparison of pharmacological and genetic inhibition of cyclooxygenase-2: effects on adult neurogenesis in the hippocampal dentate gyrus