Brain Tumor Res Treat.
2022 Jul;10(3):172-182. 10.14791/btrt.2022.0016.
Survival Outcomes and Predictors for Recurrence of Surgically Treated Brain Metastasis From Non-Small Cell Lung Cancer
- Affiliations
-
- 1Department of Neurosurgery, Korea University Guro Hospital, Korea University College of Medicine, Seoul, Korea
- 2Department of Neurological Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2532251
- DOI: http://doi.org/10.14791/btrt.2022.0016
Abstract
- Background
There are numerous factors to consider in deciding whether to undergo surgical treatment for brain metastasis from lung cancer. Herein, we aimed to analyze the survival outcome and predictors of recurrence of surgically treated brain metastasis from non-small cell lung cancer (NSCLC).
Methods
A total of 197 patients with brain metastasis from NSCLC who underwent microsurgery were included in this study.
Results
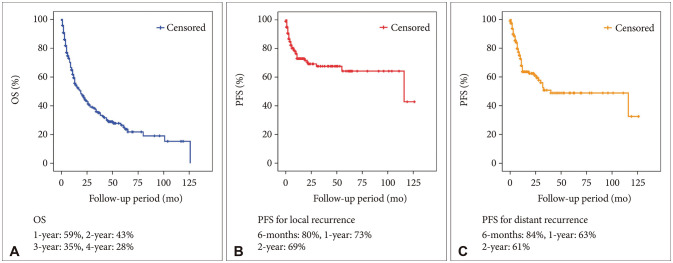
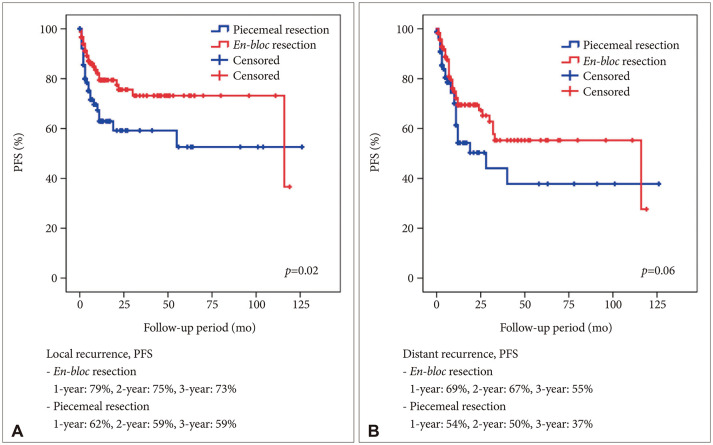
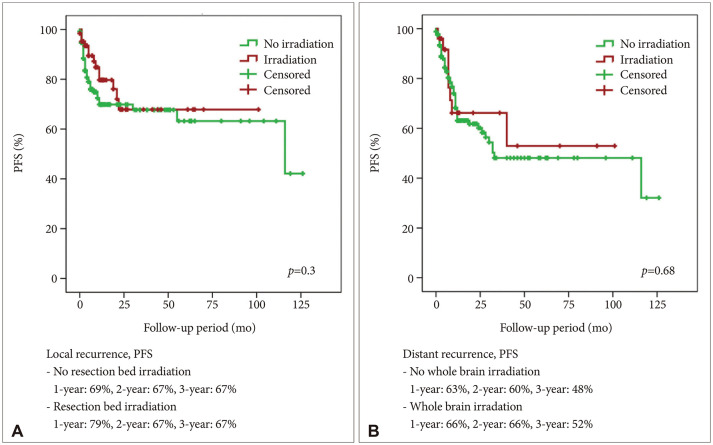
A total of 114 (57.9%) male and 83 (42.1%) female patients with a median age of 59 years (range, 27–79) was included in this study. The median follow-up period was 22.7 (range, 1–126) months. The 1-year and 2-year overall survival (OS) rates of patients with brain metastasis secondary to NSCLC were 59% and 43%, respectively. The 6-month and 1-year progression-free survival (PFS) rates of local recurrence were 80% and 73%, respectively, whereas those of distant recurrence were 84% and 63%, respectively. En-bloc resection of tumor resulted in better PFS for local recurrence (1-year PFS: 79% vs. 62%, p=0.02). Ventricular opening and direct contact between the tumor and the subarachnoid space were not associated with distal recurrence and leptomeningeal seeding. The difference in PFS of local recurrence according to adjuvant resection bed irradiation was not significant. Moreover, postoperative whole-brain irradiation did not show a significant difference in PFS of distant recurrence. In multivariate analysis, only En-bloc resection was a favorable prognostic factor for local recurrence. Contrastingly, multiple metastasis was a poor prognostic factor for distant recurrence.
Conclusion
En-bloc resection may reduce local recurrence after surgical resection. Ventricular opening and contact between the tumor and subarachnoid space did not show a statistically significant result for distant recurrence and leptomeningeal seeding. Multiple metastasis was only meaningful factor for distant recurrence.
Keyword
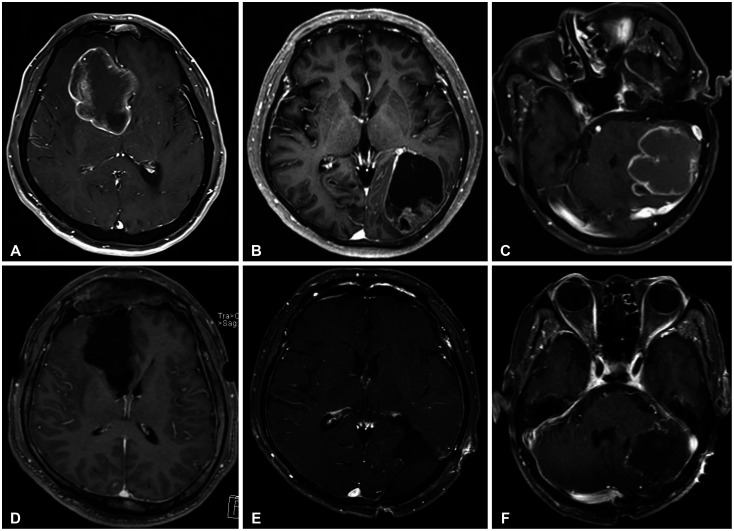
Figure
Reference
-
1. Jung KW, Won YJ, Kong HJ, Lee ES. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2016. Cancer Res Treat. 2019; 51:417–430. PMID: 30913865.2. Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004; 22:2865–2872. PMID: 15254054.3. Fox BD, Cheung VJ, Patel AJ, Suki D, Rao G. Epidemiology of metastatic brain tumors. Neurosurg Clin N Amb. 2011; 22:1–6.4. Abdallah SM, Wong A. Brain metastases in non-small-cell lung cancer: are tyrosine kinase inhibitors and checkpoint inhibitors now viable options? Curr Oncol. 2018; 25(Suppl 1):S103–S114. PMID: 29910653.5. Mulvenna P, Nankivell M, Barton R, Faivre-Finn C, Wilson P, McColl E, et al. Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ): results from a phase 3, non-inferiority, randomised trial. Lancet. 2016; 388:2004–2014. PMID: 27604504.6. Lamba N, Muskens IS, DiRisio AC, Meijer L, Briceno V, Edrees H, et al. Stereotactic radiosurgery versus whole-brain radiotherapy after intracranial metastasis resection: a systematic review and meta-analysis. Radiat Oncol. 2017; 12:106. PMID: 28646895.7. Fuentes R, Bonfill X, Exposito J. Surgery versus radiosurgery for patients with a solitary brain metastasis from non-small cell lung cancer. Cochrane Database Syst Rev. 2006; 2006:CD004840.8. Gavrilovic IT, Posner JB. Brain metastases: epidemiology and pathophysiology. J Neurooncol. 2005; 75:5–14. PMID: 16215811.9. Chon H, Yoon K, Lee D, Kwon DH, Cho YH. Single-fraction versus hypofractionated stereotactic radiosurgery for medium-sized brain metastases of 2.5 to 3 cm. J Neurooncol. 2019; 145:49–56. PMID: 31420793.10. Patel TR, Knisely JP, Chiang VL. Management of brain metastases: surgery, radiation, or both? Hematol Oncol Clin North Am. 2012; 26:933–947. PMID: 22794291.11. Yoo H, Kim YZ, Nam BH, Shin SH, Yang HS, Lee JS, et al. Reduced local recurrence of a single brain metastasis through microscopic total resection. J Neurosurg. 2009; 110:730–736. PMID: 19072310.12. Ahn MJ, Kim DW, Cho BC, Kim SW, Lee JS, Ahn JS, et al. Activity and safety of AZD3759 in EGFR-mutant non-small-cell lung cancer with CNS metastases (BLOOM): a phase 1, open-label, dose-escalation and dose-expansion study. Lancet Respir Med. 2017; 5:891–902. PMID: 29056570.13. Putz F, Weissmann T, Oft D, Schmidt MA, Roesch J, Siavooshhaghighi H, et al. FSRT vs. SRS in brain metastases—differences in local control and radiation necrosis—a volumetric study. Front Oncol. 2020; 10:559193. PMID: 33102223.14. Ahmed Z, Balagamwala E, Murphy E, Angelov L, Suh J, Lo S, et al. Postoperative stereotactic radiosurgery for resected brain metastasis. CNS Oncol. 2014; 3:199–207. PMID: 25055128.15. Chang EL, Wefel JS, Hess KR, Allen PK, Lang FF, Kornguth DG, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009; 10:1037–1044. PMID: 19801201.16. Khuntia D, Brown P, Li J, Mehta MP. Whole-brain radiotherapy in the management of brain metastasis. J Clin Oncol. 2006; 24:1295–1304. PMID: 16525185.17. Lu-Emerson C, Eichler AF. Brain metastases. Continuum (Minneap Minn). 2012; 18:295–311. PMID: 22810128.18. Zer A, Leighl N. Promising targets and current clinical trials in metastatic non-squamous NSCLC. Front Oncol. 2014; 4:329. PMID: 25505733.19. Magnuson WJ, Lester-Coll NH, Wu AJ, Yang TJ, Lockney NA, Gerber NK, et al. Management of brain metastases in tyrosine kinase inhibitor-naïve epidermal growth factor receptor-mutant non-small-cell lung cancer: a retrospective multi-institutional analysis. J Clin Oncol. 2017; 35:1070–1077. PMID: 28113019.20. Sagerup CM, Småstuen M, Johannesen TB, Helland Å, Brustugun OT. Sex-specific trends in lung cancer incidence and survival: a population study of 40,118 cases. Thorax. 2011; 66:301–307. PMID: 21199818.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Two Cases of Cutaneous Metastasis from Small Cell Lung Cancer
- A case of leptomeningeal metastasis from adenocarcinoma of the lung improved by treatment with Gefitinib
- Pattern of Recurrence after Curative Resection of Local (Stage I and II) Non-Small Cell Lung Cancer: Difference According to the Histologic Type
- Postoperative Radiation Therapy in Resected N2 Stage Non-Small Cell Lung Cancer
- The Efficacy of Postoperative Chemotherapy for Patients with Metastatic Brain Tumors from Non-Small Cell Lung Cancer