Kosin Med J.
2022 Jun;37(2):134-139. 10.7180/kmj.22.102.
STAT3 inhibition decreases ATP-induced MUC8 gene expression in human airway epithelial cells
- Affiliations
-
- 1Department of Pediatrics, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, Changwon, Korea
- 2Department of Medical Science, Kosin University College of Medicine, Busan, Korea
- KMID: 2532043
- DOI: http://doi.org/10.7180/kmj.22.102
Abstract
- Background
Contact between the human pulmonary system and bacteria, viruses, or other pathogens can induce airway diseases. Although pathogen-induced mucus oversecretion and hyperproduction are frequently observed in the human respiratory tract, the molecular mechanisms of pathogen-induced mucus hypersecretion and overproduction remain unclear. The objective of this study was to investigate the physiological signaling mechanism of ATP-induced MUC8 gene expression in human airway epithelial cells.
Methods
Real-time reverse transcription polymerase chain reaction, a cytokine array, and a Ca2+ concentration assay were performed to investigate the ATP/P2Y2-induced MUC8 gene expression levels in human airway epithelial cells.
Results
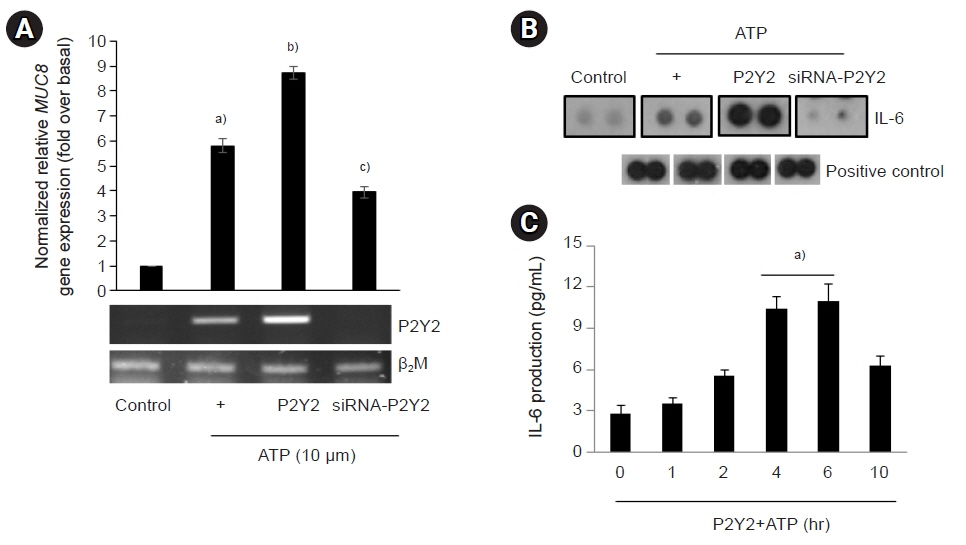
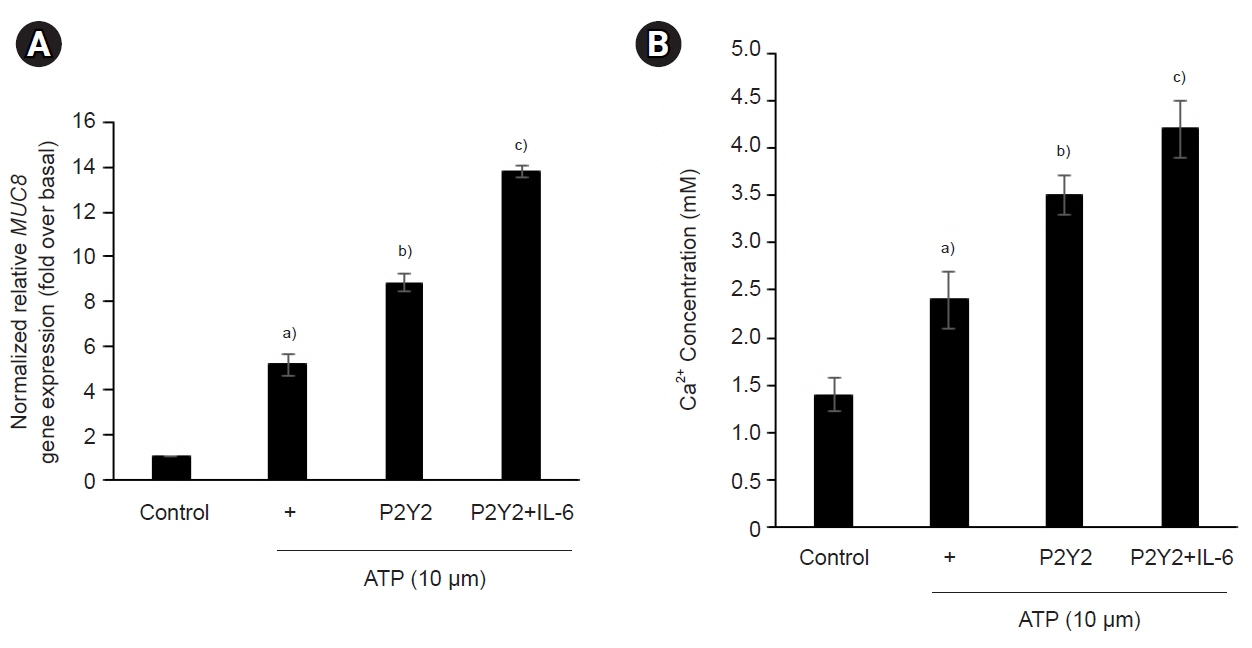
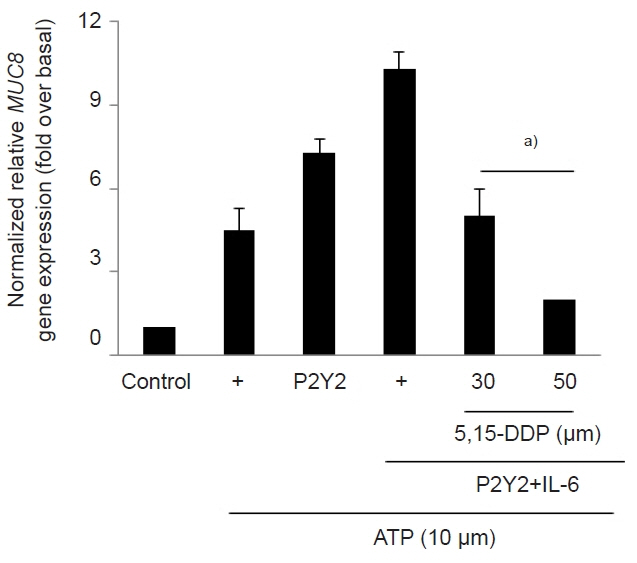
The ATP/P2Y2 complex robustly secreted interleukin (IL)-6 in a time-dependent manner, whereas siRNA-P2Y2 did not. Moreover, ATP/P2Y2 induced MUC8 gene expression. IL-6 secreted by ATP strongly elevated ATP/P2Y2-induced MUC8 gene expression compared to ATP/P2Y2. Interestingly, a specific STAT3 inhibitor, 5,15-DPP, dramatically inhibited ATP/P2Y2/IL-6-induced STAT3 phosphorylation and resulted in an approximately 5-fold decrease in MUC8 gene expression.
Conclusions
We showed that IL-6-activated STAT6 is essential for ATP/P2Y2-induced MUC8 gene expression as part of inflammatory signaling by cytokines during airway inflammation. Our results provide a new molecular understanding of the signaling mechanism of MUC8 gene expression during airway inflammation.
Keyword
Figure
Reference
-
References
1. Kim YO, Jung MJ, Choi JK, Ahn do W, Song KS. Peptidoglycan from Staphylococcus aureus increases MUC5AC gene expression via RSK1-CREB pathway in human airway epithelial cells. Mol Cells. 2011; 32:359–65.
Article2. Lee HM, Kim DH, Kim JM, Lee SH, Hwang SJ. MUC8 mucin gene up-regulation in chronic rhinosinusitis. Ann Otol Rhinol Laryngol. 2004; 113:662–6.
Article3. Shankar V, Pichan P, Eddy RL Jr, Tonk V, Nowak N, Sait SN, et al. Chromosomal localization of a human mucin gene (MUC8) and cloning of the cDNA corresponding to the carboxy terminus. Am J Respir Cell Mol Biol. 1997; 16:232–41.
Article4. Cha HJ, Jung MS, Ahn do W, Choi JK, Ock MS, Kim KS, et al. Silencing of MUC8 by siRNA increases P2Y₂-induced airway inflammation. Am J Physiol Lung Cell Mol Physiol. 2015; 308:L495–502.5. Eltzschig HK, Sitkovsky MV, Robson SC. Purinergic signaling during inflammation. N Engl J Med. 2012; 367:2322–33.
Article6. Schenk U, Westendorf AM, Radaelli E, Casati A, Ferro M, Fumagalli M, et al. Purinergic control of T cell activation by ATP released through pannexin-1 hemichannels. Sci Signal. 2008; 1:ra6.
Article7. Jeong JY, Kim J, Kim B, Kim J, Shin Y, Kim J, et al. IL-1ra secreted by ATP-induced P2Y2 negatively regulates MUC5AC overproduction via PLCβ3 during airway inflammation. Mediators Inflamm. 2016; 2016:7984853.8. Vanderstocken G, Van de Paar E, Robaye B, di Pietrantonio L, Bondue B, Boeynaems JM, et al. Protective role of P2Y2 receptor against lung infection induced by pneumonia virus of mice. PLoS One. 2012; 7:e50385.
Article9. Gavino AC, Nahmod K, Bharadwaj U, Makedonas G, Tweardy DJ. STAT3 inhibition prevents lung inflammation, remodeling, and accumulation of Th2 and Th17 cells in a murine asthma model. Allergy. 2016; 71:1684–92.
Article10. Doganci A, Eigenbrod T, Krug N, De Sanctis GT, Hausding M, Erpenbeck VJ, et al. The IL-6R alpha chain controls lung CD4+CD25+ Treg development and function during allergic airway inflammation in vivo. J Clin Invest. 2005; 115:313–25.11. Finotto S, Eigenbrod T, Karwot R, Boross I, Doganci A, Ito H, et al. Local blockade of IL-6R signaling induces lung CD4+ T cell apoptosis in a murine model of asthma via regulatory T cells. Int Immunol. 2007; 19:685–93.
Article12. Kim JI. Analysis of an EGFR mutation by PNA clamping method in lung carcinoid tumors. Kosin Med J. 2015; 30:141–7.
Article13. Kong EH, Ma SY, Jeong JY, Kim KH. Effects of L-ascorbic acid on the production of pro-inflammatory and anti-inflammatory cytokines in C57BL/6 mouse splenocytes. Kosin Med J. 2015; 30:41–9.
Article14. Rincon M, Irvin CG. Role of IL-6 in asthma and other inflammatory pulmonary diseases. Int J Biol Sci. 2012; 8:1281–90.
Article15. Yokoyama A, Kohno N, Fujino S, Hamada H, Inoue Y, Fujioka S, et al. Circulating interleukin-6 levels in patients with bronchial asthma. Am J Respir Crit Care Med. 1995; 151:1354–8.
Article16. Tillie-Leblond I, Pugin J, Marquette CH, Lamblin C, Saulnier F, Brichet A, et al. Balance between proinflammatory cytokines and their inhibitors in bronchial lavage from patients with status asthmaticus. Am J Respir Crit Care Med. 1999; 159:487–94.
Article17. Simeone-Penney MC, Severgnini M, Tu P, Homer RJ, Mariani TJ, Cohn L, et al. Airway epithelial STAT3 is required for allergic inflammation in a murine model of asthma. J Immunol. 2007; 178:6191–9.
Article18. Kasembeli MM, Bharadwaj U, Robinson P, Tweardy DJ. Contribution of STAT3 to inflammatory and fibrotic diseases and prospects for its targeting for treatment. Int J Mol Sci. 2018; 19:2299.
Article19. Schaunaman N, Dimasuay KG, Kraft M, Chu HW. Tollip interaction with STAT3: a novel mechanism to regulate human airway epithelial responses to type 2 cytokines. Respir Res. 2022; 23:31.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Roxithromycin Suppresses MUC5B/8 Mucin Genes and Mucin Production in Airway Epithelial Cells
- Inhibitory Effect of 15-Deoxy-Delta(12,14)-Prostaglandin J(2) on the MUC8 Expression in the Human Airway Epithelial Cells
- Delphinidin Inhibits LPS-Induced MUC8 and MUC5B Expression Through Toll-like Receptor 4-Mediated ERK1/2 and p38 MAPK in Human Airway Epithelial Cells
- Rhinovirus-Induced Mucin Gene Expression in Airway Epithelial Cells
- The Role of p38 MAP Kinase Signal Transduction in the Induction of MUC 8 Gene Expression in Normal Human Nasal Epithelial Cells