Anesth Pain Med.
2022 Apr;17(2):191-198. 10.17085/apm.21096.
Adverse events of sugammadex that occurred in a Korean population
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Eulji University School of Medicine, Daejeon, Korea
- KMID: 2531695
- DOI: http://doi.org/10.17085/apm.21096
Abstract
- Background
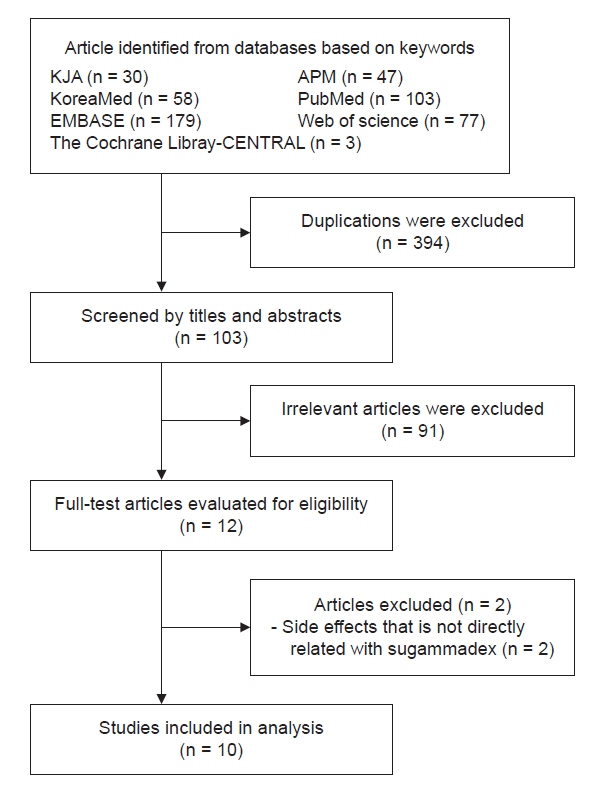
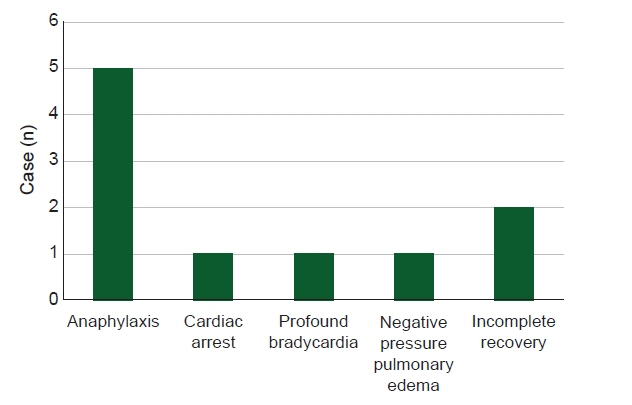
With increasing use, the incidence of adverse events associated with sugammadex, a neuromuscular blockade reverser, is increasing. This study aimed to identify and analyze cases of adverse events caused by sugammadex reported in Korean population. Methods: Out of a total of 12 cases detected using various keywords in the Korean Journal of Anesthesia, Anesthesia and Pain Medicine (Seoul), KoreaMed, PubMed, EMBASE, Web of Science, and The Cochrane Library-CENTRAL from 2013 to December 2020, 10 cases directly associated with sugammadex were selected. Results: Adverse events included five cases of anaphylaxis, one case of cardiac arrest, one case of profound bradycardia, one case of negative pressure pulmonary edema, and two cases of incomplete recovery. Three patients had American Society of Anesthesiologists physical status ≥ 3, two had emergency surgery, and two had a history of allergic reaction. Neuromuscular monitoring was applied in nine cases. The average dose of sugammadex was 2.87 mg/kg, and there were six cases in which one full vial was used, regardless of the state of neuromuscular recovery. Sugammadex was administered immediately after surgery in two cases, at train of four (TOF) 0 in four cases, at TOF 3 in one case, and after evaluation of the clinical signs only with no neuromuscular monitoring in one case. Conclusions: Even with neuromuscular monitoring, an excessive dose of sugammadex was observed. Given that adverse events tend to occur within 10 min of administration, continuous monitoring is important even after administration.
Figure
Reference
-
1. Jones RS, Auer U, Mosing M. Reversal of neuromuscular block in companion animals. Vet Anaesth Analg. 2015; 42:455–71.
Article2. Heeley E, Riley J, Layton D, Wilton LV, Shakir SA. Prescription-event monitoring and reporting of adverse drug reactions. Lancet. 2001; 358:1872–3.
Article3. Hwang MH, Won YJ, Lee IO, Koo EH, Jung WJ. A suspected case of sugammadex-induced anaphylactic shock: a case report. Anesth Pain Med. 2015; 10:288–90.
Article4. Koo BS, Lee SJ, Na HW, Kang WB, Kim SH. A suspected sugammadex-induced anaphylactic shock - a case report -. Anesth Pain Med. 2019; 14:294–8.
Article5. Choi SC, Han S, Kwak J, Lee JY. Anaphylaxis induced by sugammadex and sugammadex-rocuronium complex - a case report -. Korean J Anesthesiol. 2020; 73:342–6.
Article6. Kim GH, Choi WS, Kim JE, Yun MJ, Koo MS, Kwon M, et al. Anaphylactic shock after sugammadex administration, induced by formation of a sugammadex-rocuronium complex - a case report -. Korean J Anesthesiol. 2019; 72:495–9.
Article7. Yoo JH, Kim SI, Ok SY, Park SY, Cho A, Han YM, et al. Suspected anaphylactic reaction associated with sugammadex: a case report. Korean J Anesthesiol. 2016; 69:413–6.
Article8. Ko MJ, Kim YH, Kang E, Lee BC, Lee S, Jung JW. Cardiac arrest after sugammadex administration in a patient with variant angina: a case report. Korean J Anesthesiol. 2016; 69:514–7.
Article9. Choi YJ, Park JW, Kim SH, Jung KT. Sugammadex associated profound bradycardia and sustained hypotension in patient with the slow recovery of neuromuscular blockade - a case report -. Anesth Pain Med. 2019; 14:299–304.
Article10. Lee JH, Lee JH, Lee MH, Cho HO, Park SE. Postoperative negative pressure pulmonary edema following repetitive laryngospasm even after reversal of neuromuscular blockade by sugammadex: a case report. Korean J Anesthesiol. 2017; 70:95–9.
Article11. Chun HR, Chung J, Kim NS, Kim AJ, Kim S, Kang KS. Incomplete recovery from rocuronium-induced muscle relaxation in patients with amyotrophic lateral sclerosis using sugammadex: a case report. Medicine (Baltimore). 2020; 99:e18867.12. Kim JE, Chun HR. Rocuronium-induced neuromuscular block and sugammadex in pediatric patient with Duchenne muscular dystrophy: a case report. Medicine (Baltimore). 2017; 96:e6456.13. Iwasaki H, Kurosawa A, Iida T, Sasakawa T, Kanda H. Use of intraoperative neuromuscular monitor reduces the reversal dose of sugammadex: a single-center retrospective study. J Anesth. 2020; 34:276–80.
Article14. Kaufhold N, Schaller SJ, Stäuble CG, Baumüller E, Ulm K, Blobner M, et al. Sugammadex and neostigmine dose-finding study for reversal of residual neuromuscular block at a train-of-four ratio of 0.2 (SUNDRO20). Br J Anaesth. 2016; 116:233–40.
Article15. Iwasaki H, Renew JR, Kunisawa T, Brull SJ. Preparing for the unexpected: special considerations and complications after sugammadex administration. BMC Anesthesiol. 2017; 17:140.
Article16. Booij LH. Cyclodextrins and the emergence of sugammadex. Anaesthesia. 2009; 64 Suppl 1:31–7.
Article17. de Kam PJ, Nolte H, Good S, Yunan M, Williams-Herman DE, Burggraaf J, et al. Sugammadex hypersensitivity and underlying mechanisms: a randomised study of healthy non-anaesthetised volunteers. Br J Anaesth. 2018; 121:758–67.
Article18. Takazawa T, Mitsuhata H, Mertes PM. Sugammadex and rocuronium-induced anaphylaxis. J Anesth. 2016; 30:290–7.
Article19. Min KC, Bondiskey P, Schulz V, Woo T, Assaid C, Yu W, et al. Hypersensitivity incidence after sugammadex administration in healthy subjects: a randomised controlled trial. Br J Anaesth. 2018; 121:749–57.20. IA Sanoja and KS Toth. Profound bradycardia and cardiac arrest after sugammadex administration in a previously healthy patient: A Case Report. A A Pract. 2019; 12:22–4.
Article21. Bhavani SS. Severe bradycardia and asystole after sugammadex. Br J Anaesth. 2018; 121:95–6.
Article22. Suzuki M, Inagi T, Kikutani T, Mishima T, Bito H. Negative pressure pulmonary edema after reversing rocuronium-induced neuromuscular blockade by sugammadex. Case Rep Anesthesiol. 2014; 2014:135032.
Article23. Krodel DJ, Bittner EA, Abdulnour R, Brown R, Eikermann M. Case scenario: acute postoperative negative pressure pulmonary edema. Anesthesiology. 2010; 113:200–7.
Article24. Lee HJ, Kim KS, Kim TY, Lee JH, Jeong M. The use of 3 sugammadex out of 5 reversal of during recovery of rocuronium-induced neuromuscular blockade in a patient with post-tonsillectomy hemorrhage: a case report. Korean J Anesthesiol. 2014; 67:43–7.
Article25. Kim YB, Choi JM, Chang YJ, Choi HR, In J, Yang HS. Effects of different sugammadex doses on the train of four ratio recovery progression during rocuronium induced neuromuscular blockade in the rat phrenic nerve hemidiaphragm. Korean J Anesthesiol. 2020; 73:239–46.
Article26. Hemmerling TM, Donati F. Neuromuscular blockade at the larynx, the diaphragm and the corrugator supercilii muscle: a review. Can J Anaesth. 2003; 50:779–94.
Article27. Eikermann M, Groeben H, Hüsing J, Peters J. Accelerometry of adductor pollicis muscle predicts recovery of respiratory function from neuromuscular blockade. Anesthesiology. 2003; 98:1333–7.28. Gurunathan U, Kunju SM, Stanton LML. Use of sugammadex in patients with neuromuscular disorders: a systematic review of case reports. BMC Anesthesiol. 2019; 19:213.
Article29. Van Gestel L, Cammu G. Is the effect of sugammadex always rapid in onset? Acta Anaesthesiol Belg. 2013; 64:41–7.30. de Boer HD, Carlos RV, Brull SJ. Is lower-dose sugammadex a cost-saving strategy for reversal of deep neuromuscular block? Facts and fiction. BMC Anesthesiol. 2018; 18:159.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Sugammadex-induced bronchospasm: a case report
- The potential risks of sugammadex
- Anaphylaxis induced by sugammadex and sugammadex-rocuronium complex -a case report-
- What we need to know and do on sugammadex usage in pregnant and lactating women and those on hormonal contraceptives
- Advantages and pitfalls of clinical application of sugammadex