Endocrinol Metab.
2022 Jun;37(3):432-443. 10.3803/EnM.2021.1336.
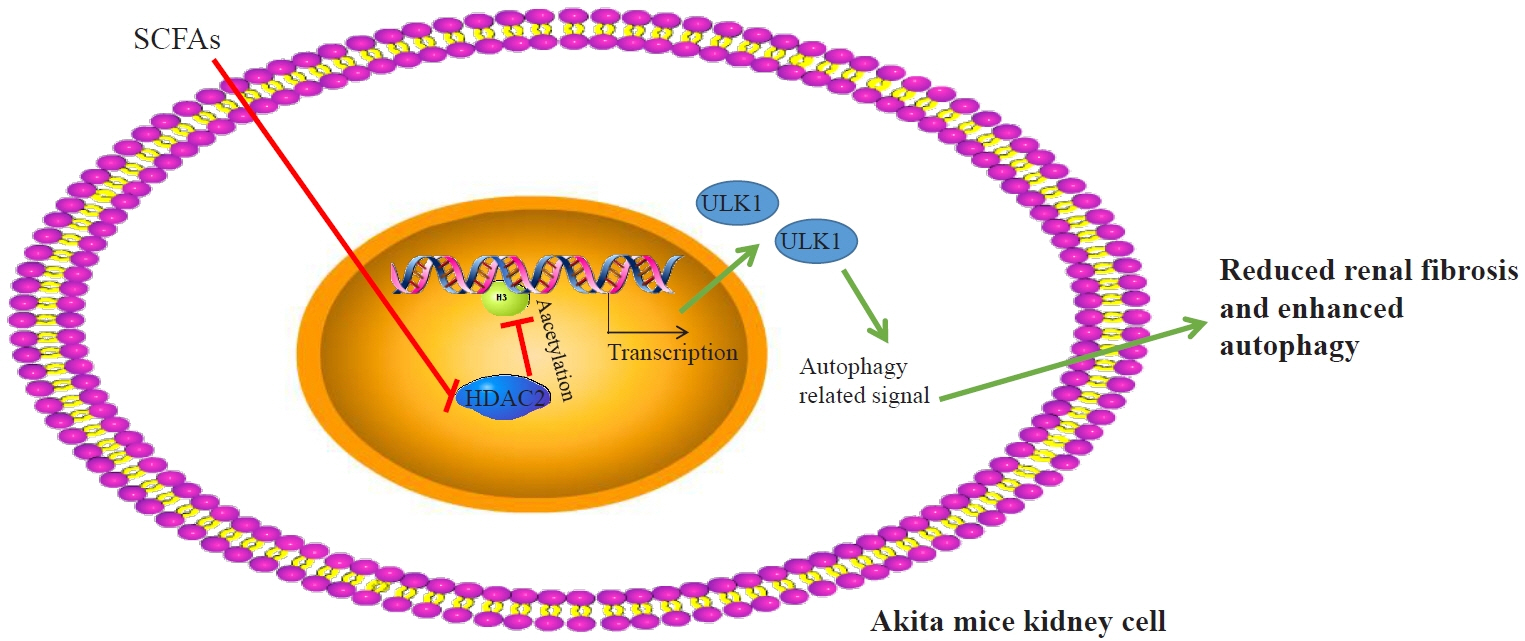
Short-Chain Fatty Acids Attenuate Renal Fibrosis and Enhance Autophagy of Renal Tubular Cells in Diabetic Mice Through the HDAC2/ULK1 Axis
- Affiliations
-
- 1Department of Gastroenterology, Kidney Disease and Hemodialysis Center, Shaanxi Provincial People’s Hospital, Xi’an, China
- 2Kidney Disease and Hemodialysis Center, Shaanxi Provincial People’s Hospital, Xi’an, China
- KMID: 2531387
- DOI: http://doi.org/10.3803/EnM.2021.1336
Abstract
- Background
This study investigated the effect of short-chain fatty acids (SCFAs) on diabetes in a mouse model.
Methods
Autophagy in Akita mice and streptozocin (STZ)-induced diabetic C57BL/6 mice was determined by Western blots and immunohistochemistry (IHC). Western blots, IHC, hematoxylin and eosin staining, Masson staining, periodic acid-Schiff staining, and picrosirius red staining were conducted to detect whether autophagy and renal function improved in Akita mice and STZ-induced diabetic C57BL/6 mice after treatment of SCFAs. Western blots, IHC, and chromatin immunoprecipitation were performed to determine whether SCFAs affected diabetic mice via the histone deacetylase (HDAC2)/unc-51 like autophagy activating kinase 1 (ULK1) axis. Diabetic mice with kidney-specific knockout of HDAC2 were constructed, and IHC, Masson staining, and Western blots were carried out to detect whether the deletion of endogenous HDAC2 contributed to the improvement of autophagy and renal fibrosis in diabetic mice.
Results
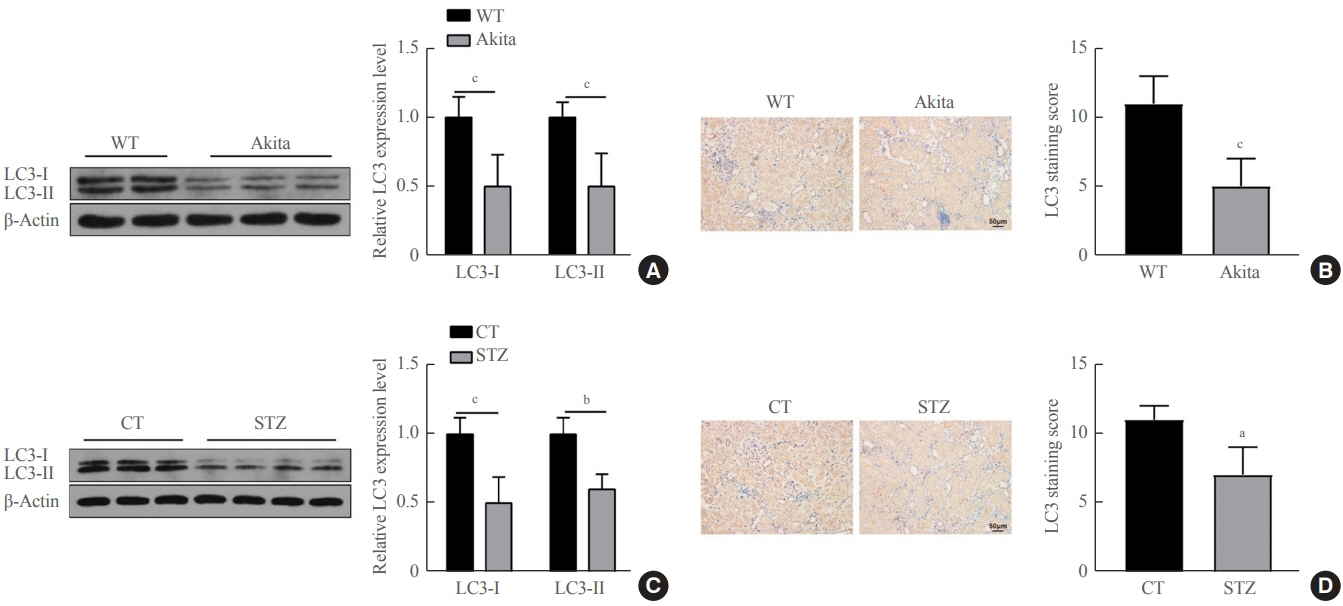
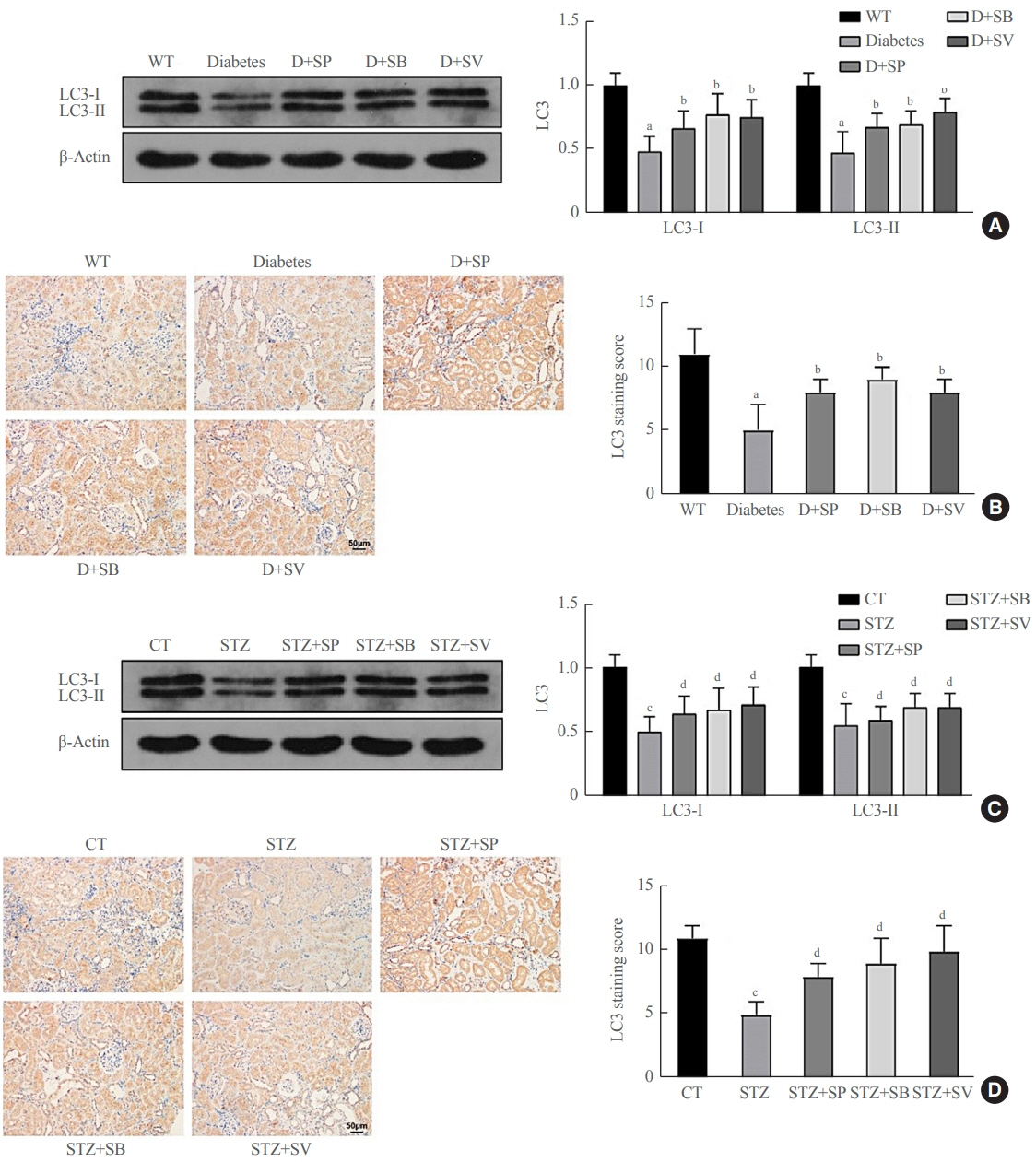
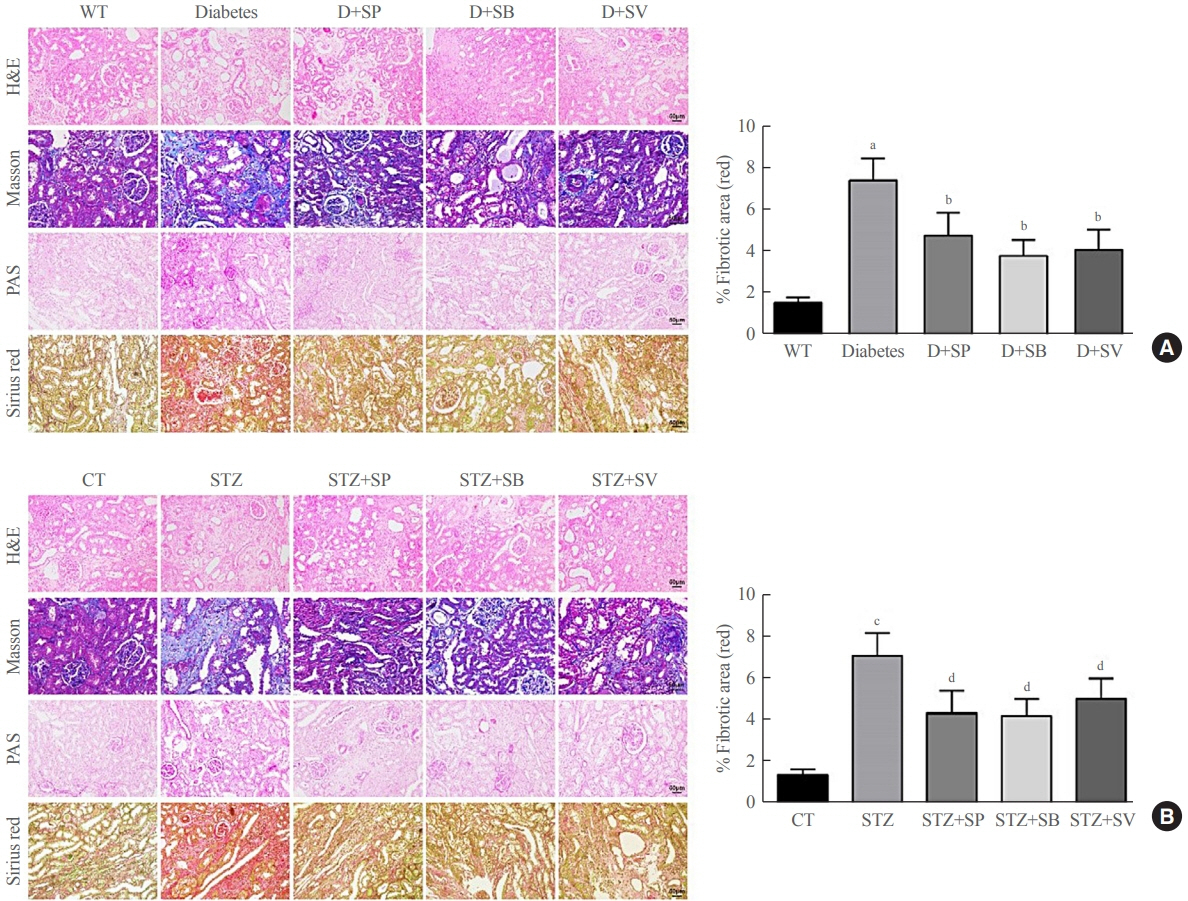
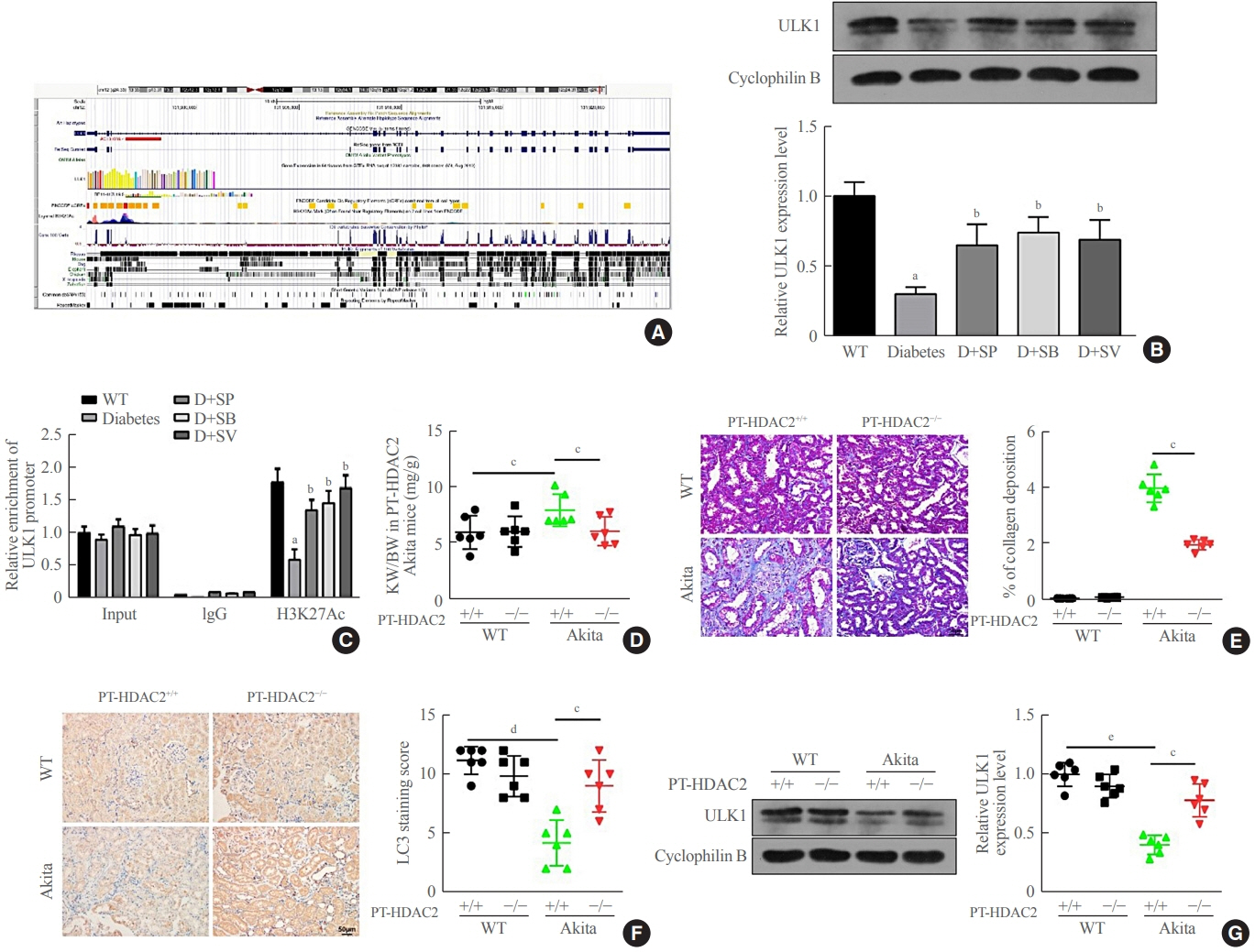
Reduced autophagy and severe fibrosis were observed in Akita mice and STZ-induced diabetic C57BL/6 mice. Increased autophagy and reduced renal cell fibrosis were found in SCFA-treated Akita diabetic mice and STZ-induced diabetic C57BL/6 mice. Diabetic mice treated with SCFAs had lower HDAC2 expression and more enriched binding of ULK1 promoter sequences to H3K27Ac. Endogenous knockout of HDAC2 caused enhanced autophagy and decreased renal fibrosis in diabetic mice treated with SCFAs.
Conclusion
SCFAs enhanced autophagy of renal tubular cells and attenuated renal fibrosis in diabetic mice through the HDAC2/ULK1 axis.
Figure
Reference
-
1. ValdezGuerrero AS, Quintana-Perez JC, Arellano-Mendoza MG, Castaneda-Ibarra FJ, Tamay-Cach F, Aleman-Gonzalez-Duhart D. Diabetic retinopathy: important biochemical alterations and the main treatment strategies. Can J Diabetes. 2021; 45:504–11.
Article2. Muriach M, Flores-Bellver M, Romero FJ, Barcia JM. Diabetes and the brain: oxidative stress, inflammation, and autophagy. Oxid Med Cell Longev. 2014; 2014:102158.
Article3. Aw W, Fukuda S. Understanding the role of the gut ecosystem in diabetes mellitus. J Diabetes Investig. 2018; 9:5–12.
Article4. Ma Z, Li L, Livingston MJ, Zhang D, Mi Q, Zhang M, et al. p53/microRNA-214/ULK1 axis impairs renal tubular autophagy in diabetic kidney disease. J Clin Invest. 2020; 130:5011–26.
Article5. Lin YC, Chang YH, Yang SY, Wu KD, Chu TS. Update of pathophysiology and management of diabetic kidney disease. J Formos Med Assoc. 2018; 117:662–75.
Article6. Barlow AD, Thomas DC. Autophagy in diabetes: β-cell dysfunction, insulin resistance, and complications. DNA Cell Biol. 2015; 34:252–60.
Article7. Xu BH, Sheng J, You YK, Huang XR, Ma R, Wang Q, et al. Deletion of Smad3 prevents renal fibrosis and inflammation in type 2 diabetic nephropathy. Metabolism. 2020; 103:154013.
Article8. Kobayashi M, Mikami D, Kimura H, Kamiyama K, Morikawa Y, Yokoi S, et al. Short-chain fatty acids, GPR41 and GPR43 ligands, inhibit TNF-α-induced MCP-1 expression by modulating p38 and JNK signaling pathways in human renal cortical epithelial cells. Biochem Biophys Res Commun. 2017; 486:499–505.
Article9. Mikami D, Kobayashi M, Uwada J, Yazawa T, Kamiyama K, Nishimori K, et al. Short-chain fatty acid mitigates adenine-induced chronic kidney disease via FFA2 and FFA3 pathways. Biochim Biophys Acta Mol Cell Biol Lipids. 2020; 1865:158666.
Article10. Li YJ, Chen X, Kwan TK, Loh YW, Singer J, Liu Y, et al. Dietary fiber protects against diabetic nephropathy through short-chain fatty acid-mediated activation of G protein-coupled receptors GPR43 and GPR109A. J Am Soc Nephrol. 2020; 31:1267–81.
Article11. Kasubuchi M, Hasegawa S, Hiramatsu T, Ichimura A, Kimura I. Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation. Nutrients. 2015; 7:2839–49.
Article12. Wen L, Wong FS. Dietary short-chain fatty acids protect against type 1 diabetes. Nat Immunol. 2017; 18:484–6.
Article13. Zhu L, Sha L, Li K, Wang Z, Wang T, Li Y, et al. Dietary flaxseed oil rich in omega-3 suppresses severity of type 2 diabetes mellitus via anti-inflammation and modulating gut microbiota in rats. Lipids Health Dis. 2020; 19:20.
Article14. Andrade-Oliveira V, Amano MT, Correa-Costa M, Castoldi A, Felizardo RJ, de Almeida DC, et al. Gut bacteria products prevent AKI induced by ischemia-reperfusion. J Am Soc Nephrol. 2015; 26:1877–88.
Article15. Hsiao YP, Chen HL, Tsai JN, Lin MY, Liao JW, Wei MS, et al. Administration of Lactobacillus reuteri combined with Clostridium butyricum attenuates cisplatin-induced renal damage by gut microbiota reconstitution, increasing butyric acid production, and suppressing renal inflammation. Nutrients. 2021; 13:2792.
Article16. Wang H, Qian J, Zhao X, Xing C, Sun B. β-Aminoisobutyric acid ameliorates the renal fibrosis in mouse obstructed kidneys via inhibition of renal fibroblast activation and fibrosis. J Pharmacol Sci. 2017; 133:203–13.
Article17. Khan S, Jena G, Tikoo K. Sodium valproate ameliorates diabetes-induced fibrosis and renal damage by the inhibition of histone deacetylases in diabetic rat. Exp Mol Pathol. 2015; 98:230–9.
Article18. Tan J, McKenzie C, Potamitis M, Thorburn AN, Mackay CR, Macia L. The role of short-chain fatty acids in health and disease. Adv Immunol. 2014; 121:91–119.
Article19. Silva LG, Ferguson BS, Avila AS, Faciola AP. Sodium propionate and sodium butyrate effects on histone deacetylase (HDAC) activity, histone acetylation, and inflammatory gene expression in bovine mammary epithelial cells. J Anim Sci. 2018; 96:5244–52.20. Orinska Z, Maurer M, Mirghomizadeh F, Bulanova E, Metz M, Nashkevich N, et al. IL-15 constrains mast cell-dependent antibacterial defenses by suppressing chymase activities. Nat Med. 2007; 13:927–34.
Article21. Guan JS, Haggarty SJ, Giacometti E, Dannenberg JH, Joseph N, Gao J, et al. HDAC2 negatively regulates memory formation and synaptic plasticity. Nature. 2009; 459:55–60.
Article22. Rankin EB, Tomaszewski JE, Haase VH. Renal cyst development in mice with conditional inactivation of the von Hippel-Lindau tumor suppressor. Cancer Res. 2006; 66:2576–83.
Article23. Riser BL, Najmabadi F, Perbal B, Rambow JA, Riser ML, Sukowski E, et al. CCN3/CCN2 regulation and the fibrosis of diabetic renal disease. J Cell Commun Signal. 2010; 4:39–50.
Article24. Ma Q, Li Y, Li P, Wang M, Wang J, Tang Z, et al. Research progress in the relationship between type 2 diabetes mellitus and intestinal flora. Biomed Pharmacother. 2019; 117:109138.
Article25. Sakai S, Yamamoto T, Takabatake Y, Takahashi A, Namba-Hamano T, Minami S, et al. Proximal tubule autophagy differs in type 1 and 2 diabetes. J Am Soc Nephrol. 2019; 30:929–45.
Article26. Gasaly N, de Vos P, Hermoso MA. Impact of bacterial metabolites on gut barrier function and host immunity: a focus on bacterial metabolism and its relevance for intestinal inflammation. Front Immunol. 2021; 12:658354.
Article27. Wang S, Lv D, Jiang S, Jiang J, Liang M, Hou F, et al. Quantitative reduction in short-chain fatty acids, especially butyrate, contributes to the progression of chronic kidney disease. Clin Sci (Lond). 2019; 133:1857–70.
Article28. Zheng C, Huang L, Luo W, Yu W, Hu X, Guan X, et al. Inhibition of STAT3 in tubular epithelial cells prevents kidney fibrosis and nephropathy in STZ-induced diabetic mice. Cell Death Dis. 2019; 10:848.
Article29. Ghorbani P, Santhakumar P, Hu Q, Djiadeu P, Wolever TM, Palaniyar N, et al. Short-chain fatty acids affect cystic fibrosis airway inflammation and bacterial growth. Eur Respir J. 2015; 46:1033–45.
Article30. Ohira H, Tsutsui W, Fujioka Y. Are short chain fatty acids in gut microbiota defensive players for inflammation and atherosclerosis? J Atheroscler Thromb. 2017; 24:660–72.
Article31. Lammert C, Shin A, Xu H, Hemmerich C, O’Connell TM, Chalasani N. Short-chain fatty acid and fecal microbiota profiles are linked to fibrosis in primary biliary cholangitis. FEMS Microbiol Lett. 2021; 368:fnab038.
Article32. Pluznick JL. Gut microbiota in renal physiology: focus on short-chain fatty acids and their receptors. Kidney Int. 2016; 90:1191–8.
Article33. Makkar R, Behl T, Arora S. Role of HDAC inhibitors in diabetes mellitus. Curr Res Transl Med. 2020; 68:45–50.
Article34. Ho RH, Chan J, Fan H, Kioh D, Lee BW, Chan E. In silico and in vitro interactions between short chain fatty acids and human histone deacetylases. Biochemistry. 2017; 56:4871–8.
Article35. Yu X, Shahir AM, Sha J, Feng Z, Eapen B, Nithianantham S, et al. Short-chain fatty acids from periodontal pathogens suppress histone deacetylases, EZH2, and SUV39H1 to promote Kaposi’s sarcoma-associated herpesvirus replication. J Virol. 2014; 88:4466–79.
Article36. Donohoe DR, Holley D, Collins LB, Montgomery SA, Whitmore AC, Hillhouse A, et al. A gnotobiotic mouse model demonstrates that dietary fiber protects against colorectal tumorigenesis in a microbiota- and butyrate-dependent manner. Cancer Discov. 2014; 4:1387–97.
Article37. Christensen DP, Dahllof M, Lundh M, Rasmussen DN, Nielsen MD, Billestrup N, et al. Histone deacetylase (HDAC) inhibition as a novel treatment for diabetes mellitus. Mol Med. 2011; 17:378–90.
Article38. Khan S, Jena GB. Protective role of sodium butyrate, a HDAC inhibitor on beta-cell proliferation, function and glucose homeostasis through modulation of p38/ERK MAPK and apoptotic pathways: study in juvenile diabetic rat. Chem Biol Interact. 2014; 213:1–12.
Article39. Wang Y, Chen Q, Jiao F, Shi C, Pei M, Wang L, et al. Histone deacetylase 2 regulates ULK1 mediated pyroptosis during acute liver failure by the K68 acetylation site. Cell Death Dis. 2021; 12:55.
Article40. Zhou C, Li L, Li T, Sun L, Yin J, Guan H, et al. SCFAs induce autophagy in intestinal epithelial cells and relieve colitis by stabilizing HIF-1α. J Mol Med (Berl). 2020; 98:1189–202.
Article41. Iannucci LF, Sun J, Singh BK, Zhou J, Kaddai VA, Lanni A, et al. Short chain fatty acids induce UCP2-mediated autophagy in hepatic cells. Biochem Biophys Res Commun. 2016; 480:461–7.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Sulforaphane Ameliorates Diabetes-Induced Renal Fibrosis through Epigenetic Up-Regulation of BMP-7
- Aging-related renal fibrosis was alleviated via conserving mitochondrial function and autophagy in NLRP3 KO mice
- Omega-3 Polyunsaturated Fatty Acids May Attenuate Streptozotocin-Induced Pancreatic beta-Cell Death via Autophagy Activation in Fat1 Transgenic Mice
- Urine-derived stem cell attenuated renal fibrosis via Klotho activation
- Autophagy: A Novel Therapeutic Target for Diabetic Nephropathy