Diabetes Metab J.
2021 Nov;45(6):909-920. 10.4093/dmj.2020.0168.
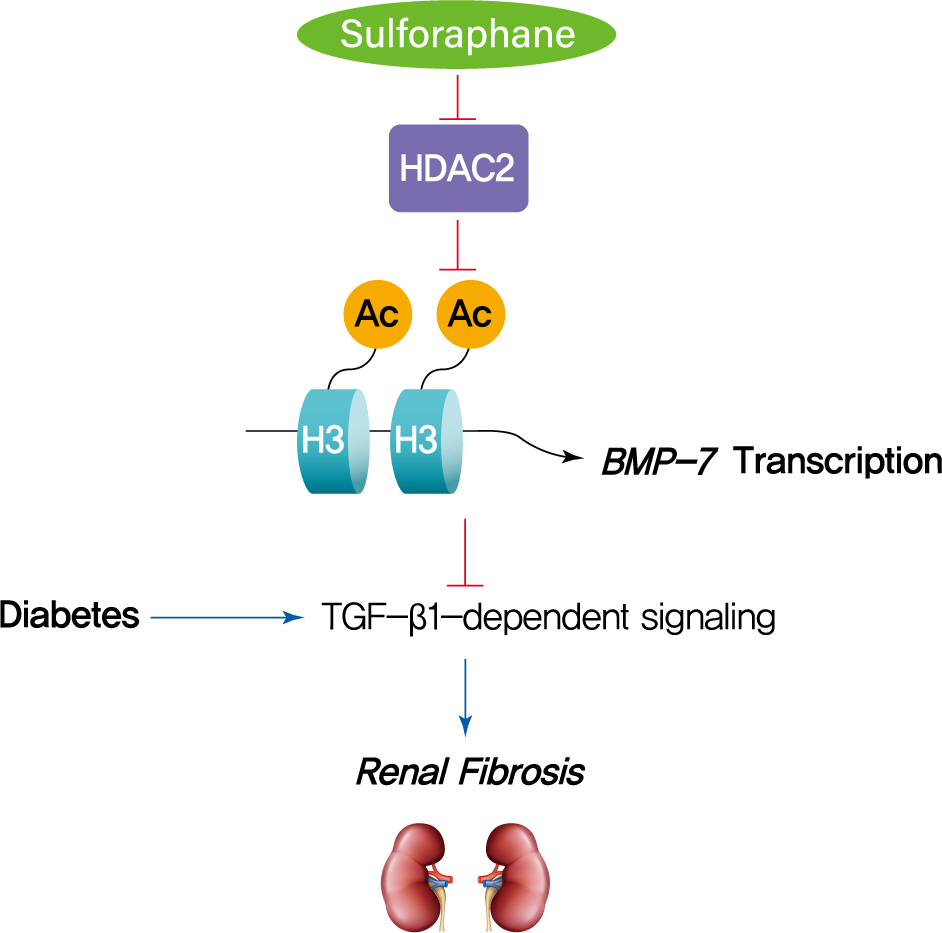
Sulforaphane Ameliorates Diabetes-Induced Renal Fibrosis through Epigenetic Up-Regulation of BMP-7
- Affiliations
-
- 1Department of Nephrology, the First Hospital of Jilin University, Changchun, China
- 2Department of Obstetrics and Gynecology, the First Hospital of Jilin University, Changchun, China
- KMID: 2522730
- DOI: http://doi.org/10.4093/dmj.2020.0168
Abstract
- Background
The dietary agent sulforaphane (SFN) has been reported to reduce diabetes-induced renal fibrosis, as well as inhibit histone deacetylase (HDAC) activity. Bone morphologic protein 7 (BMP-7) has been shown to reduce renal fibrosis induced by transforming growth factor-beta1. The aim of this study was to investigate the epigenetic effect of SFN on BMP-7 expression in diabetes-induced renal fibrosis.
Methods
Streptozotocin (STZ)-induced diabetic mice and age-matched controls were subcutaneously injected with SFN or vehicle for 4 months to measure the in vivo effects of SFN on the kidneys. The human renal proximal tubular (HK11) cell line was used to mimic diabetic conditions in vitro. HK11 cells were transfected to over-express HDAC2 and treated with high glucose/palmitate (HG/Pal) to explore the epigenetic modulation of BMP-7 in SFN-mediated protection against HG/Pal-induced renal fibrosis.
Results
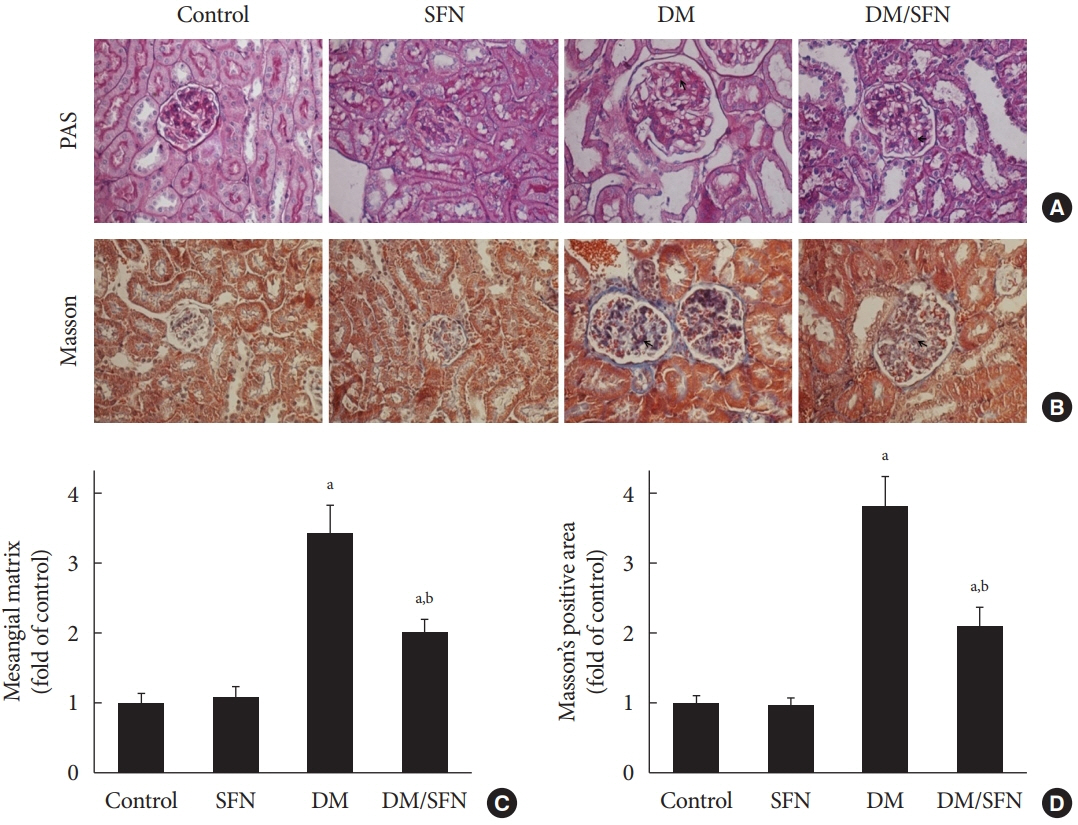
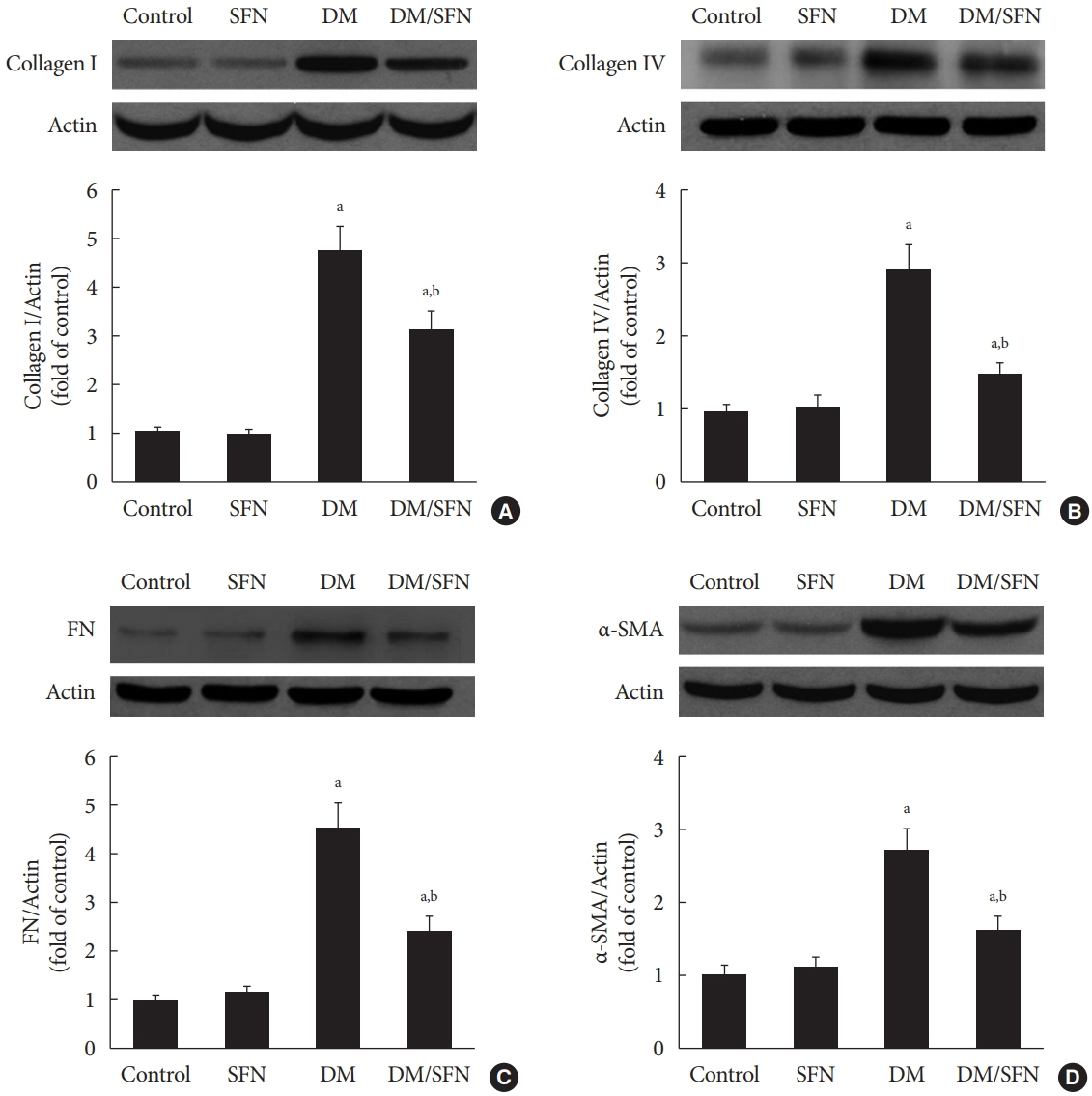
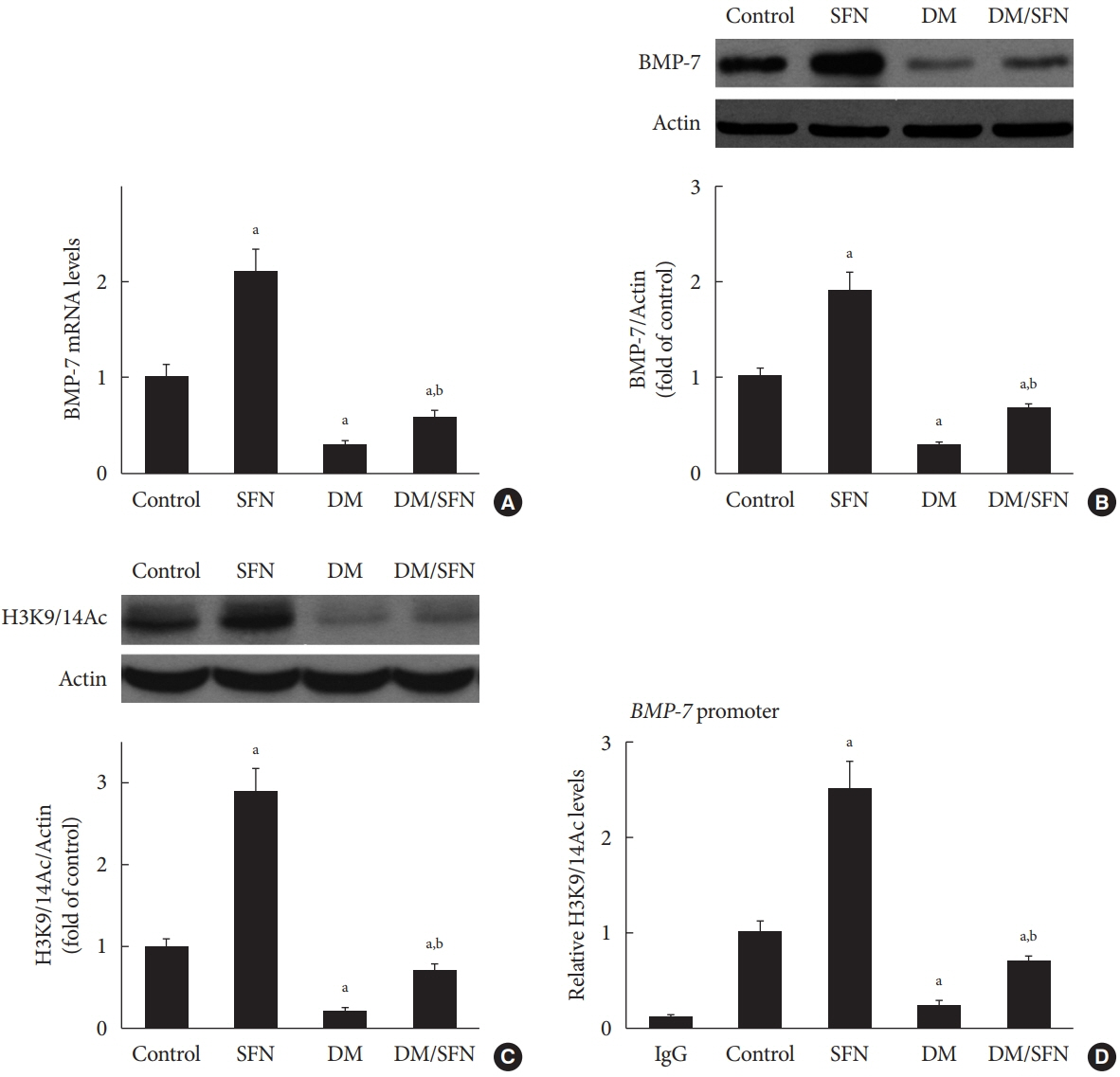
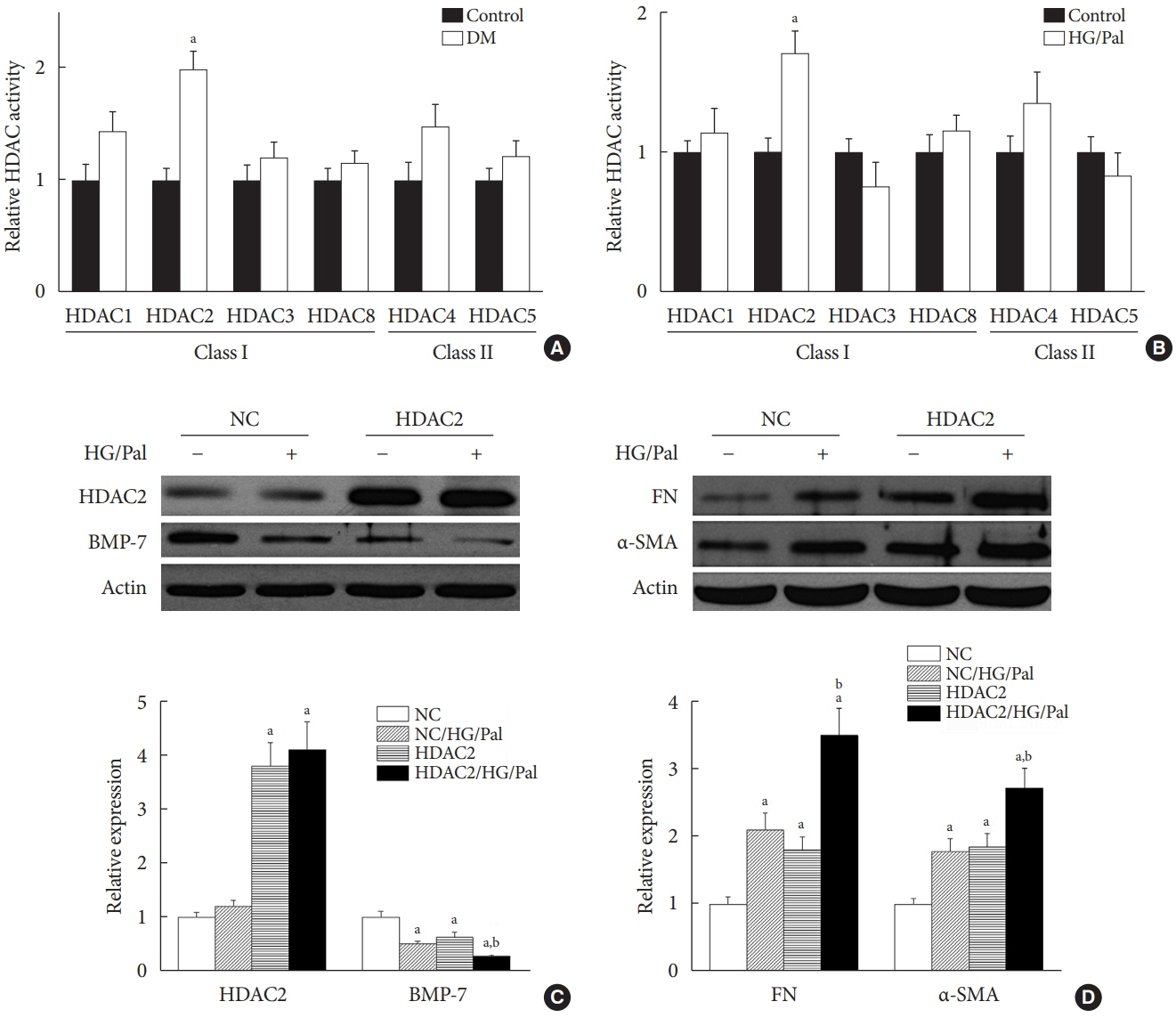
SFN significantly attenuated diabetes-induced renal fibrosis in vivo. Among all of the HDACs we detected, HDAC2 activity was markedly elevated in the STZ-induced diabetic kidneys and HG/Pal-treated HK11 cells. SFN inhibited the diabetes-induced increase in HDAC2 activity which was associated with histone acetylation and transcriptional activation of the BMP-7 promoter. HDAC2 over-expression reduced BMP-7 expression and abolished the SFN-mediated protection against HG/Pal-induced fibrosis in vitro.
Conclusion
Our study demonstrates that the HDAC inhibitor SFN protects against diabetes-induced renal fibrosis through epigenetic up-regulation of BMP-7.
Figure
Reference
-
1. Sun XY, Qin HJ, Zhang Z, Xu Y, Yang XC, Zhao DM, et al. Valproate attenuates diabetic nephropathy through inhibition of endoplasmic reticulum stress induced apoptosis. Mol Med Rep. 2016; 13:661–8.2. Hakami NY, Dusting GJ, Peshavariya HM. Trichostatin A, a histone deacetylase inhibitor suppresses NADPH oxidase 4-derived redox signalling and angiogenesis. J Cell Mol Med. 2016; 20:1932–44.3. Chen Y, Du J, Zhao YT, Zhang L, Lv G, Zhuang S, et al. Histone deacetylase (HDAC) inhibition improves myocardial function and prevents cardiac remodeling in diabetic mice. Cardiovasc Diabetol. 2015; 14:99.
Article4. Singh RS, Chaudhary DK, Mohan A, Kumar P, Chaturvedi CP, Ecelbarger CM, et al. Greater efficacy of atorvastatin versus a non-statin lipid-lowering agent against renal injury: potential role as a histone deacetylase inhibitor. Sci Rep. 2016; 6:38034.
Article5. Khan S, Jena G, Tikoo K. Sodium valproate ameliorates diabetes-induced fibrosis and renal damage by the inhibition of histone deacetylases in diabetic rat. Exp Mol Pathol. 2015; 98:230–9.
Article6. Lee E, Song MJ, Lee HA, Kang SH, Kim M, Yang EK, et al. Histone deacetylase inhibitor, CG200745, attenuates cardiac hypertrophy and fibrosis in DOCA-induced hypertensive rats. Korean J Physiol Pharmacol. 2016; 20:477–85.
Article7. Zhu P, Xing S, Xu Q, Xie T, Gao Y, He Z. Effect and mechanism of inhibition of lipopolysaccharide-induced pulmonary fibrosis by butyric acid. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2016; 28:8–14.8. Martin SL, Kala R, Tollefsbol TO. Mechanisms for the inhibition of colon cancer cells by sulforaphane through epigenetic modulation of microRNA-21 and human telomerase reverse transcriptase (hTERT) down-regulation. Curr Cancer Drug Targets. 2018; 18:97–106.
Article9. Abbaoui B, Telu KH, Lucas CR, Thomas-Ahner JM, Schwartz SJ, Clinton SK, et al. The impact of cruciferous vegetable isothiocyanates on histone acetylation and histone phosphorylation in bladder cancer. J Proteomics. 2017; 156:94–103.
Article10. Kim BG, Fujita T, Stankovic KM, Welling DB, Moon IS, Choi JY, et al. Sulforaphane, a natural component of broccoli, inhibits vestibular schwannoma growth in vitro and in vivo. Sci Rep. 2016; 6:36215.
Article11. Cui W, Bai Y, Miao X, Luo P, Chen Q, Tan Y, et al. Prevention of diabetic nephropathy by sulforaphane: possible role of Nrf2 upregulation and activation. Oxid Med Cell Longev. 2012; 2012:821936.
Article12. Shang G, Tang X, Gao P, Guo F, Liu H, Zhao Z, et al. Sulforaphane attenuation of experimental diabetic nephropathy involves GSK-3 beta/Fyn/Nrf2 signaling pathway. J Nutr Biochem. 2015; 26:596–606.
Article13. Ivanac-Jankovic R, Coric M, Furic-Cunko V, Lovicic V, BasicJukic N, Kes P. BMP-7 protein expression is downregulated in human diabetic nephropathy. Acta Clin Croat. 2015; 54:164–8.14. Zeisberg M, Hanai J, Sugimoto H, Mammoto T, Charytan D, Strutz F, et al. BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat Med. 2003; 9:964–8.15. Hruska KA, Guo G, Wozniak M, Martin D, Miller S, Liapis H, et al. Osteogenic protein-1 prevents renal fibrogenesis associated with ureteral obstruction. Am J Physiol Renal Physiol. 2000; 279:F130–43.
Article16. Morrissey J, Hruska K, Guo G, Wang S, Chen Q, Klahr S. Bone morphogenetic protein-7 improves renal fibrosis and accelerates the return of renal function. J Am Soc Nephrol. 2002; 13 Suppl 1:S14–21.
Article17. Klahr S, Morrissey J, Hruska K, Wang S, Chen Q. New approaches to delay the progression of chronic renal failure. Kidney Int Suppl. 2002; 23–6.
Article18. Zeisberg M, Bottiglio C, Kumar N, Maeshima Y, Strutz F, Muller GA, et al. Bone morphogenic protein-7 inhibits progression of chronic renal fibrosis associated with two genetic mouse models. Am J Physiol Renal Physiol. 2003; 285:F1060–7.
Article19. Vukicevic S, Basic V, Rogic D, Basic N, Shih MS, Shepard A, et al. Osteogenic protein-1 (bone morphogenetic protein-7) reduces severity of injury after ischemic acute renal failure in rat. J Clin Invest. 1998; 102:202–14.
Article20. Yoshikawa M, Hishikawa K, Marumo T, Fujita T. Inhibition of histone deacetylase activity suppresses epithelial-to-mesenchymal transition induced by TGF-beta1 in human renal epithelial cells. J Am Soc Nephrol. 2007; 18:58–65.21. Manson SR, Song JB, Hruska KA, Austin PF. HDAC dependent transcriptional repression of Bmp-7 potentiates TGF-β mediated renal fibrosis in obstructive uropathy. J Urol. 2014; 191:242–52.22. Marumo T, Hishikawa K, Yoshikawa M, Fujita T. Epigenetic regulation of BMP7 in the regenerative response to ischemia. J Am Soc Nephrol. 2008; 19:1311–20.
Article23. Guerrero-Beltran CE, Calderon-Oliver M, Pedraza-Chaverri J, Chirino YI. Protective effect of sulforaphane against oxidative stress: recent advances. Exp Toxicol Pathol. 2012; 64:503–8.24. Song Y, Li C, Cai L. Fluvastatin prevents nephropathy likely through suppression of connective tissue growth factor-mediated extracellular matrix accumulation. Exp Mol Pathol. 2004; 76:66–75.
Article25. Cui W, Li B, Bai Y, Miao X, Chen Q, Sun W, et al. Potential role for Nrf2 activation in the therapeutic effect of MG132 on diabetic nephropathy in OVE26 diabetic mice. Am J Physiol Endocrinol Metab. 2013; 304:E87–99.
Article26. Tan G, Xiao Q, Song H, Ma F, Xu F, Peng D, et al. Type I IFN augments IL-27-dependent TRIM25 expression to inhibit HBV replication. Cell Mol Immunol. 2018; 15:272–81.
Article27. Theocharis AD, Skandalis SS, Gialeli C, Karamanos NK. Extracellular matrix structure. Adv Drug Deliv Rev. 2016; 97:4–27.
Article28. Noh H, Oh EY, Seo JY, Yu MR, Kim YO, Ha H, et al. Histone deacetylase-2 is a key regulator of diabetes- and transforming growth factor-beta1-induced renal injury. Am J Physiol Renal Physiol. 2009; 297:F729–39.29. Choi SY, Kee HJ, Jin L, Ryu Y, Sun S, Kim GR, et al. Inhibition of class IIa histone deacetylase activity by gallic acid, sulforaphane, TMP269, and panobinostat. Biomed Pharmacother. 2018; 101:145–54.
Article30. Manson SR, Niederhoff RA, Hruska KA, Austin PF. The BMP-7-Smad1/5/8 pathway promotes kidney repair after obstruction induced renal injury. J Urol. 2011; 185:2523–30.
Article31. Anderson L, Gomes MR, daSilva LF, Pereira ADSA, Mourao MM, Romier C, et al. Histone deacetylase inhibition modulates histone acetylation at gene promoter regions and affects genome-wide gene transcription in Schistosoma mansoni. PLoS Negl Trop Dis. 2017; 11:e0005539.
Article32. Pan B, Quan J, Liu L, Xu Z, Zhu J, Huang X, et al. Epigallocatechin gallate reverses cTnI-low expression-induced age-related heart diastolic dysfunction through histone acetylation modification. J Cell Mol Med. 2017; 21:2481–90.
Article33. Kong L, Wu H, Zhou W, Luo M, Tan Y, Miao L, et al. Sirtuin 1: a target for kidney diseases. Mol Med. 2015; 21:87–97.
Article34. Meng XM, Chung AC, Lan HY. Role of the TGF-β/BMP-7/Smad pathways in renal diseases. Clin Sci (Lond). 2013; 124:243–54.
Article35. Manson SR, Niederhoff RA, Hruska KA, Austin PF. Endogenous BMP-7 is a critical molecular determinant of the reversibility of obstruction-induced renal injuries. Am J Physiol Renal Physiol. 2011; 301:F1293–302.
Article36. Wang SN, Lapage J, Hirschberg R. Loss of tubular bone morphogenetic protein-7 in diabetic nephropathy. J Am Soc Nephrol. 2001; 12:2392–9.
Article37. Wang S, de Caestecker M, Kopp J, Mitu G, Lapage J, Hirschberg R. Renal bone morphogenetic protein-7 protects against diabetic nephropathy. J Am Soc Nephrol. 2006; 17:2504–12.
Article38. Zheng H, Whitman SA, Wu W, Wondrak GT, Wong PK, Fang D, et al. Therapeutic potential of Nrf2 activators in streptozotocin-induced diabetic nephropathy. Diabetes. 2011; 60:3055–66.
Article39. Wu H, Kong L, Cheng Y, Zhang Z, Wang Y, Luo M, et al. Metallothionein plays a prominent role in the prevention of diabetic nephropathy by sulforaphane via up-regulation of Nrf2. Free Radic Biol Med. 2015; 89:431–42.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antifibrotic Effect of BMP-7 in the Peritoneum and the Mechanism
- Epigenetic modifications and diabetic nephropathy
- Pathogenesis and management of renal fibrosis induced by unilateral ureteral obstruction
- Fibroblast Growth Factor 21 Attenuates Diabetes-Induced Renal Fibrosis by Negatively Regulating TGF-β-p53-Smad2/3-Mediated Epithelial-to-Mesenchymal Transition via Activation of AKT
- Sulforaphane Inhibits ICAM-1 Expression and Monocyte Adhesion in Human Bladder Cancer T24 Cells