Cancer Res Treat.
2022 Jul;54(3):894-906. 10.4143/crt.2021.854.
Genomic Sequencing for Bladder Urothelial Carcinoma and Its Clinical Implications for Immunotherapy
- Affiliations
-
- 1Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 2Department of Pathology and Translational Genomics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 3Department of Urology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2531335
- DOI: http://doi.org/10.4143/crt.2021.854
Abstract
- Purpose
This study aimed to explore the genomic and transcriptomic landscape of bladder cancer (BC) and its implication for treatment with an immune checkpoint inhibitor (ICI).
Materials and Methods
We analyzed whole-exome and -transcriptome sequences of tumor samples from 64 BC patients who underwent surgical resection with either transurethral resection or radical cystectomy. For exploratory purposes, programmed death-ligand 1 (PD-L1) expression was evaluated in a subset of patients (n=57) including those treated with ICI (n=8).
Results
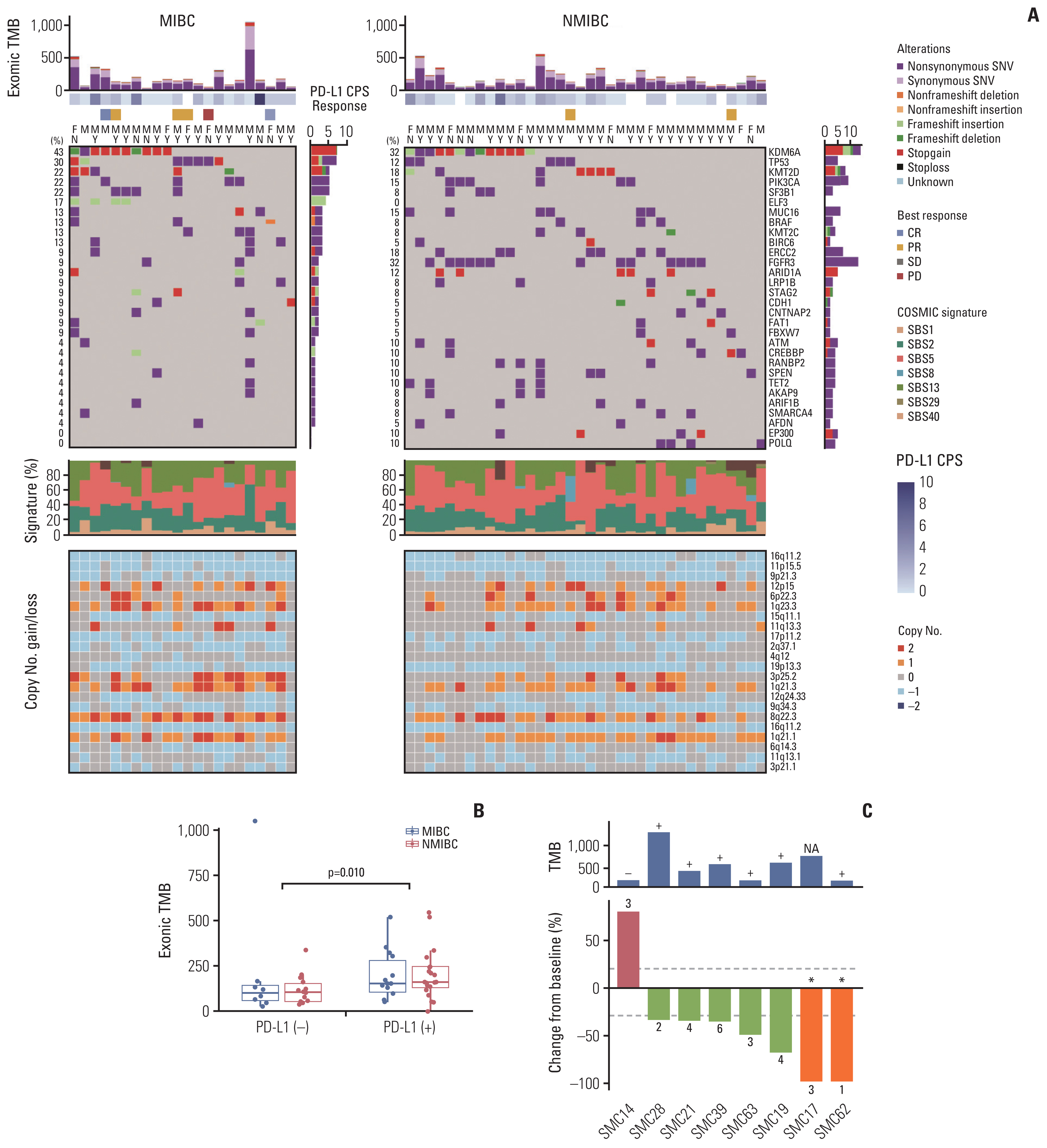
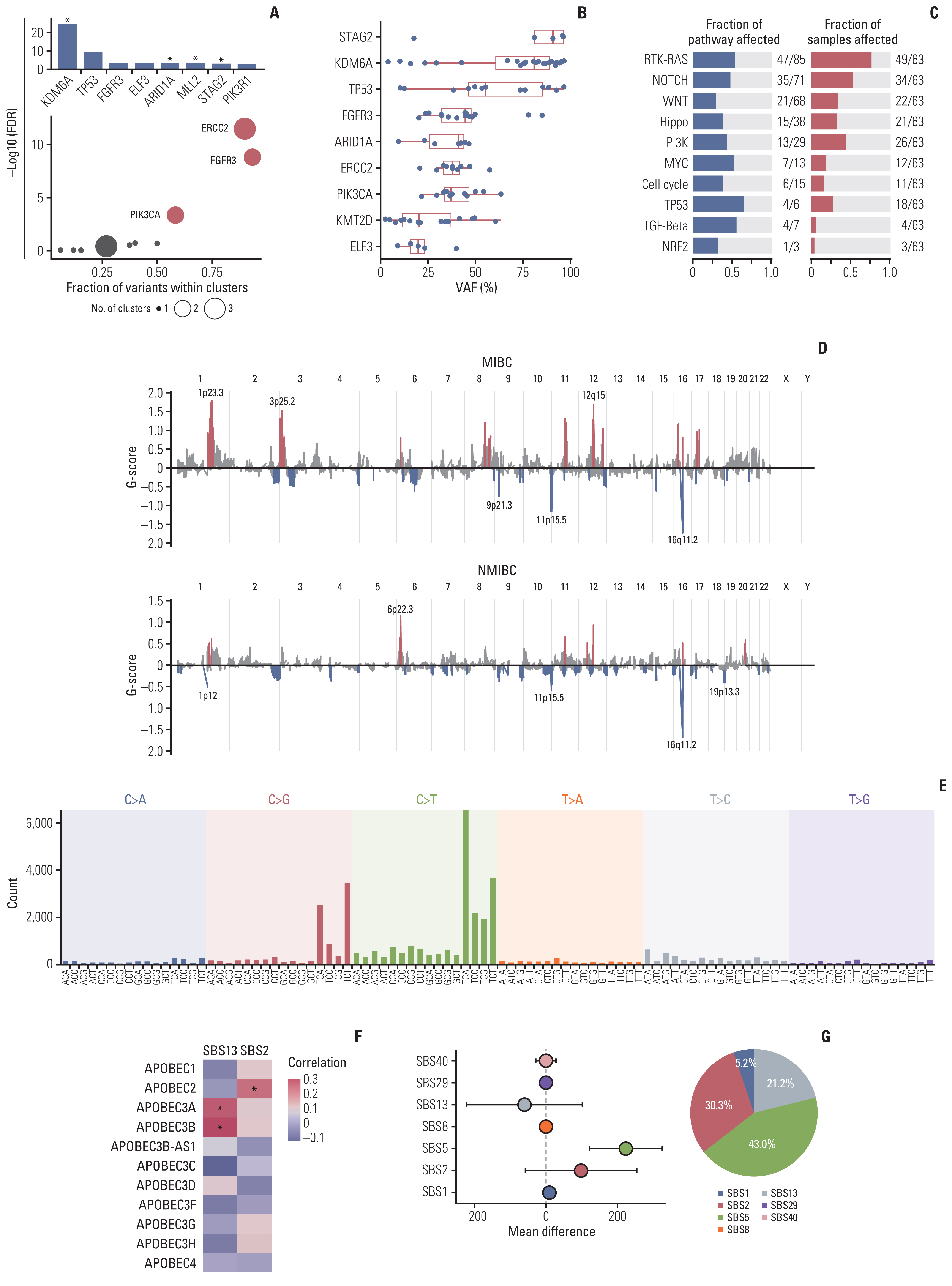
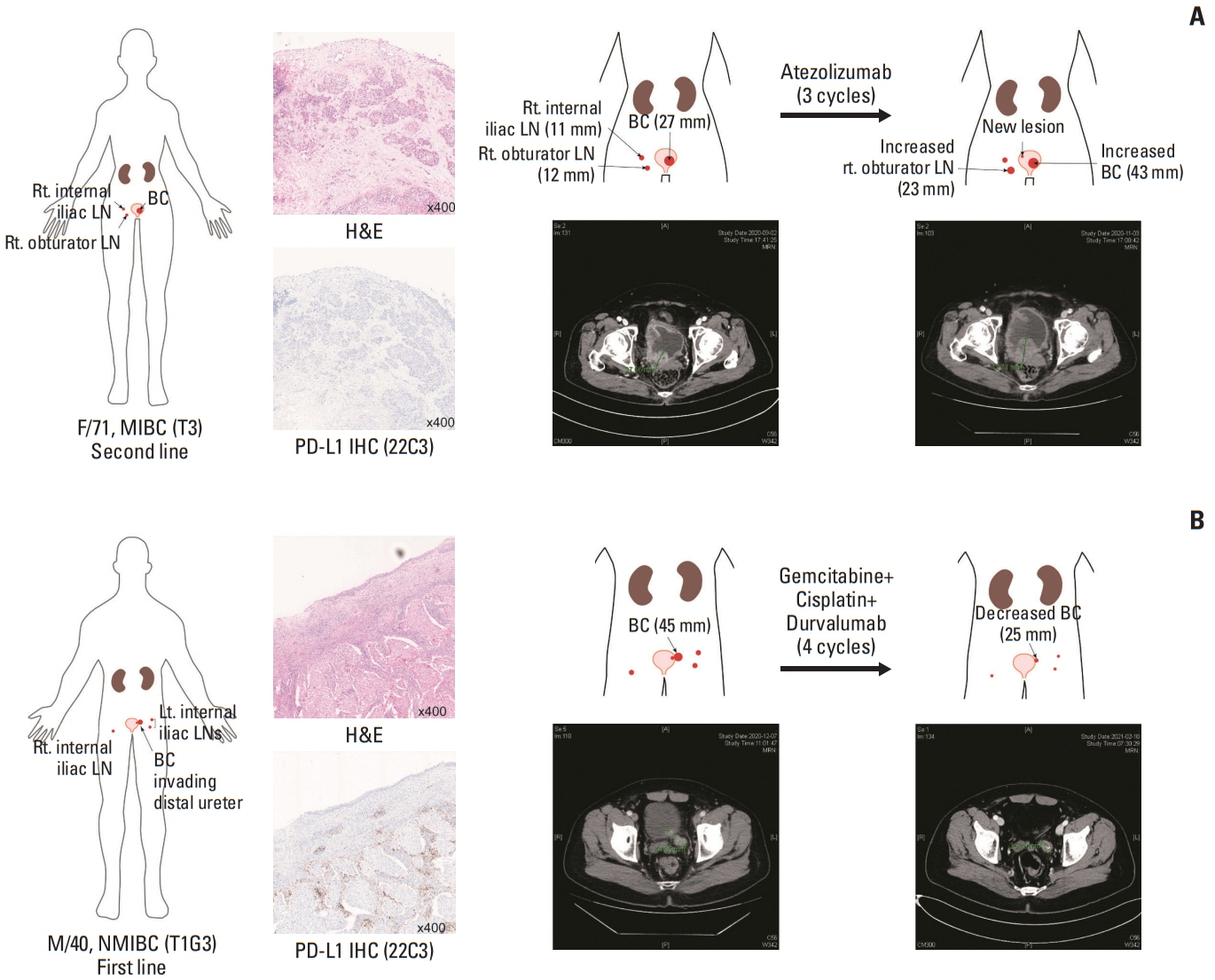
We identified frequent molecular dysregulations in chromatin regulatory genes (KDM6A, ARID1A, MLL2, and STAG2) and recurrent copy number alterations. Thirty-five samples (54.7%) were PD-L1–positive (PD-L1 combined positive score ≥ 1) with a significantly higher exonic tumor mutational burden (TMB) compared to PD-L1–negative BC samples (p=0.010). We observed that various immune-responsive pathways, including the PD-L1 signaling pathway, were enriched significantly in PD-L1–positive BCs. Interestingly, genes in the CTLA4 pathway were enriched significantly in PD-L1–positive BC as well. Among eight patients who received ICI, progressive disease was confirmed in one patient, whose tumor had low exonic TMB, negative PD-L1 status, and a relatively colder microenvironment.
Conclusion
Gaining new insights into the molecular landscape of BC will improve treatment strategies. Our analysis suggests a rationale for studying dual checkpoint inhibition against BC.
Keyword
Figure
Reference
-
References
1. Witjes JA, Bruins HM, Cathomas R, Comperat EM, Cowan NC, Gakis G, et al. European Association of Urology guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2020 guidelines. Eur Urol. 2021; 79:82–104.2. Patel VG, Oh WK, Galsky MD. Treatment of muscle-invasive and advanced bladder cancer in 2020. CA Cancer J Clin. 2020; 70:404–23.
Article3. Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013; 499:214–8.
Article4. Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, et al. Signatures of mutational processes in human cancer. Nature. 2013; 500:415–21.5. Aggen DH, Drake CG. Biomarkers for immunotherapy in bladder cancer: a moving target. J Immunother Cancer. 2017; 5:94.
Article6. Cancer Genome Atlas Research Network. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014; 507:315–22.7. Robertson AG, Kim J, Al-Ahmadie H, Bellmunt J, Guo G, Cherniack AD, et al. Comprehensive molecular characterization of muscle-invasive bladder cancer. Cell. 2017; 171:540–56.8. Kim ST, Cristescu R, Bass AJ, Kim KM, Odegaard JI, Kim K, et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med. 2018; 24:1449–58.
Article9. Yarchoan M, Albacker LA, Hopkins AC, Montesion M, Murugesan K, Vithayathil TT, et al. PD-L1 expression and tumor mutational burden are independent biomarkers in most cancers. JCI Insight. 2019; 4:e126908.
Article10. Lenis AT, Lec PM, Chamie K, Mshs MD. Bladder cancer: a review. JAMA. 2020; 324:1980–91.11. Gondkar K, Patel K, Krishnappa S, Patil A, Nair B, Sundaram GM, et al. E74 like ETS transcription factor 3 (ELF3) is a negative regulator of epithelial-mesenchymal transition in bladder carcinoma. Cancer Biomark. 2019; 25:223–32.
Article12. Gui Y, Guo G, Huang Y, Hu X, Tang A, Gao S, et al. Frequent mutations of chromatin remodeling genes in transitional cell carcinoma of the bladder. Nat Genet. 2011; 43:875–8.
Article13. Tamborero D, Gonzalez-Perez A, Lopez-Bigas N. OncodriveCLUST: exploiting the positional clustering of somatic mutations to identify cancer genes. Bioinformatics. 2013; 29:2238–44.
Article14. Sanchez-Vega F, Mina M, Armenia J, Chatila WK, Luna A, La KC, et al. Oncogenic signaling pathways in the Cancer Genome Atlas. Cell. 2018; 173:321–37.15. Alexandrov LB, Kim J, Haradhvala NJ, Huang MN, Tian Ng AW, Wu Y, et al. The repertoire of mutational signatures in human cancer. Nature. 2020; 578:94–101.
Article16. Powles T, van der Heijden MS, Castellano D, Galsky MD, Loriot Y, Petrylak DP, et al. Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced or metastatic urothelial carcinoma (DANUBE): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2020; 21:1574–88.17. van Dijk N, Gil-Jimenez A, Silina K, Hendricksen K, Smit LA, de Feijter JM, et al. Preoperative ipilimumab plus nivolumab in locoregionally advanced urothelial cancer: the NABUCCO trial. Nat Med. 2020; 26:1839–44.
Article18. Hedegaard J, Lamy P, Nordentoft I, Algaba F, Hoyer S, Ulhoi BP, et al. Comprehensive transcriptional analysis of early-stage urothelial carcinoma. Cancer Cell. 2016; 30:27–42.
Article19. Hurst CD, Alder O, Platt FM, Droop A, Stead LF, Burns JE, et al. Genomic subtypes of non-invasive bladder cancer with distinct metabolic profile and female gender bias in KDM6A mutation frequency. Cancer Cell. 2017; 32:701–15.
Article20. Gajewski TF. The next hurdle in cancer immunotherapy: overcoming the non-T-cell-inflamed tumor microenvironment. Semin Oncol. 2015; 42:663–71.
Article21. Sweis RF, Spranger S, Bao R, Paner GP, Stadler WM, Steinberg G, et al. Molecular drivers of the non-T-cell-inflamed tumor microenvironment in urothelial bladder cancer. Cancer Immunol Res. 2016; 4:563–8.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Tuberculous Prostatic Abscess Following Intravesical Bacillus Calmette-Guerin Instillation
- Seminal Vesicle Involvement by Urothelial Carcinoma in Situ of the Bladder with Mucosal Spread Pattern: A Case Report
- The Development of Antibody-Drug Conjugates for Urothelial Carcinoma Treatment
- High-Grade Urothelial Carcinoma of the Bladder in a Child
- Urothelial Carcinoma in a 17-year-old Boy