Cancer Res Treat.
2022 Jul;54(3):882-893. 10.4143/crt.2021.642.
Next-generation Proteomics-Based Discovery, Verification, and Validation of Urine Biomarkers for Bladder Cancer Diagnosis
- Affiliations
-
- 1Department of Urology, Asan Medicine Center, Seoul, Korea
- 2Department of Urology, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
- 3Proteomics Core Facility, Seoul National University Hospital Biomedical research institute, Seoul, Korea
- KMID: 2531334
- DOI: http://doi.org/10.4143/crt.2021.642
Abstract
- Purpose
We aimed to identify, verify, and validate a multiplex urinary biomarker-based prediction model for diagnosis and surveillance of urothelial carcinoma of bladder, using high-throughput proteomics methods.
Materials and Methods
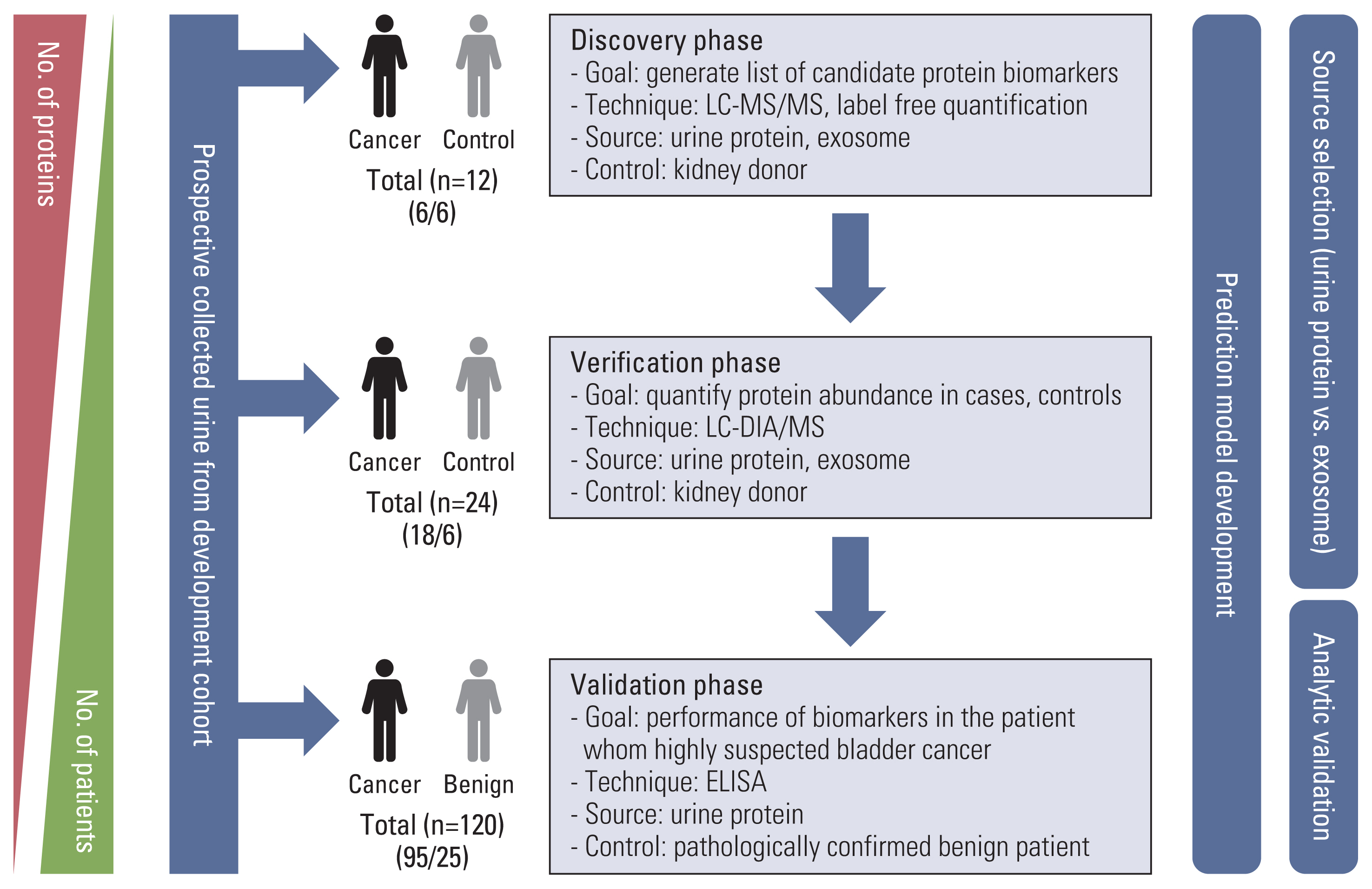
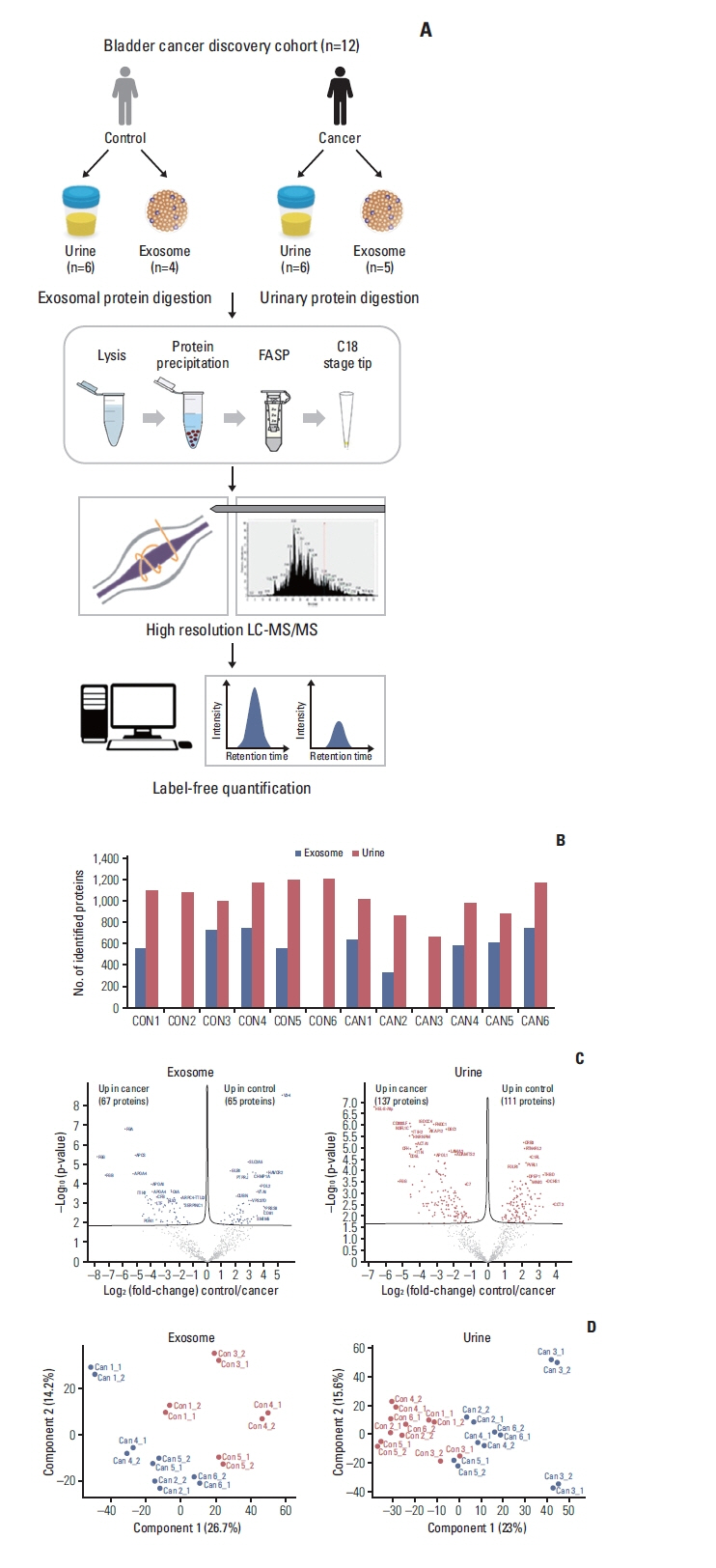
Label-free quantification of data-dependent and data-independent acquisition of 12 and 24 individuals was performed in each of the discovery and verification phases using mass spectrometry, simultaneously using urinary exosome and proteins. Based on five scoring system based on proteomics data and statistical methods, we selected eight proteins. Enzyme-linked immunosorbent assay on urine from 120 patients with bladder mass lesions used for validation. Using multivariable logistic regression, we selected final candidate models for predicting bladder cancer.
Results
Comparing the discovery and verification cohorts, 38% (50/132 exosomal differentially expressed proteins [DEPs]) and 44% (109/248 urinary DEPs) are consistent at statistically significance, respectively. The 20 out of 50 exosome proteins and 27 out of 109 urinary proteins were upregulated in cancer patients. From eight selected proteins, we developed two diagnostic models for bladder cancer. The area under the receiver operating characteristic curve (AUROC) of two models were 0.845 and 0.842, which outperformed AUROC of urine cytology.
Conclusion
The results showed that the two diagnostic models developed here were more accurate than urine cytology. We successfully developed and validated a multiplex urinary protein-based prediction, which will have wide applications for the rapid diagnosis of urothelial carcinoma of the bladder. External validation for this biomarker panel in large population is required.
Figure
Reference
-
References
1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015; 136:E359–86.
Article2. Kamat AM, Hahn NM, Efstathiou JA, Lerner SP, Malmstrom PU, Choi W, et al. Bladder cancer. Lancet. 2016; 388:2796–810.
Article3. Babjuk M, Burger M, Comperat EM, Gontero P, Mostafid AH, Palou J, et al. European Association of Urology guidelines on non-muscle-invasive Bladder Cancer (TaT1 and carcinoma In situ): 2019 update. Eur Urol. 2019; 76:639–57.4. Chang SS, Boorjian SA, Chou R, Clark PE, Daneshmand S, Konety BR, et al. Diagnosis and treatment of non-muscle invasive bladder cancer: AUA/SUO guideline. J Urol. 2016; 196:1021–9.
Article5. Soria F, Gurioli A, Peraldo F, Oderda M, Giona S, Ambrosini E, et al. Innovations in the endoscopic management of bladder cancer: is the era of white light cystoscopy over. Urologia. 2013; 80(Spec 1):1–8.
Article6. Chou R, Gore JL, Buckley D, Fu R, Gustafson K, Griffin JC, et al. Urinary biomarkers for diagnosis of bladder cancer: a systematic review and meta-analysis. Ann Intern Med. 2015; 163:922–31.7. Altelaar AF, Munoz J, Heck AJ. Next-generation proteomics: towards an integrative view of proteome dynamics. Nat Rev Genet. 2013; 14:35–48.
Article8. Liu YR, Ortiz-Bonilla CJ, Lee YF. Extracellular vesicles in bladder cancer: biomarkers and beyond. Int J Mol Sci. 2018; 19:2822.
Article9. Jeong CW, Suh J, Yuk HD, Tae BS, Kim M, Keam B, et al. Establishment of the Seoul National University prospectively enrolled registry for genitourinary cancer (SUPER-GUC): a prospective, multidisciplinary, bio-bank linked cohort and research platform. Investig Clin Urol. 2019; 60:235–43.
Article10. Han D, Jin J, Woo J, Min H, Kim Y. Proteomic analysis of mouse astrocytes and their secretome by a combination of FASP and StageTip-based, high pH, reversed-phase fractionation. Proteomics. 2014; 14:1604–9.
Article11. Bruderer R, Bernhardt OM, Gandhi T, Miladinovic SM, Cheng LY, Messner S, et al. Extending the limits of quantitative proteome profiling with data-independent acquisition and application to acetaminophen-treated three-dimensional liver microtissues. Mol Cell Proteomics. 2015; 14:1400–10.
Article12. Tyanova S, Temu T, Cox J. The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. Nat Protoc. 2016; 11:2301–19.
Article13. Cox J, Neuhauser N, Michalski A, Scheltema RA, Olsen JV, Mann M. Andromeda: a peptide search engine integrated into the MaxQuant environment. J Proteome Res. 2011; 10:1794–805.
Article14. Schwanhausser B, Busse D, Li N, Dittmar G, Schuchhardt J, Wolf J, et al. Global quantification of mammalian gene expression control. Nature. 2011; 473:337–42.
Article15. Reiter L, Rinner O, Picotti P, Huttenhain R, Beck M, Brusniak MY, et al. mProphet: automated data processing and statistical validation for large-scale SRM experiments. Nat Methods. 2011; 8:430–5.
Article16. George D, Mallery P. SPSS for Windows step by step: a simple guide and reference 110 update. London: Pearson Education;2003.17. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988; 44:837–45.
Article18. Virtanen P, Gommers R, Oliphant TE, Haberland M, Reddy T, Cournapeau D, et al. SciPy 1.0: fundamental algorithms for scientific computing in Python. Nat Methods. 2020; 17:261–72.19. Abbatiello SE, Mani DR, Schilling B, Maclean B, Zimmerman LJ, Feng X, et al. Design, implementation and multisite evaluation of a system suitability protocol for the quantitative assessment of instrument performance in liquid chromatography-multiple reaction monitoring-MS (LC-MRM-MS). Mol Cell Proteomics. 2013; 12:2623–39.
Article20. D’Costa JJ, Goldsmith JC, Wilson JS, Bryan RT, Ward DG. A systematic review of the diagnostic and prognostic value of urinary protein biomarkers in urothelial bladder cancer. Bladder Cancer. 2016; 2:301–17.
Article21. Soukup V, Kalousova M, Capoun O, Sobotka R, Breyl Z, Pesl M, et al. Panel of urinary diagnostic markers for non-invasive detection of primary and recurrent urothelial urinary bladder carcinoma. Urol Int. 2015; 95:56–64.
Article22. Santoni G, Morelli MB, Amantini C, Battelli N. Urinary markers in bladder cancer: an update. Front Oncol. 2018; 8:362.
Article23. Frantzi M, Latosinska A, Fluhe L, Hupe MC, Critselis E, Kramer MW, et al. Developing proteomic biomarkers for bladder cancer: towards clinical application. Nat Rev Urol. 2015; 12:317–30.
Article24. Linden M, Segersten U, Runeson M, Wester K, Busch C, Pettersson U, et al. Tumour expression of bladder cancer-associated urinary proteins. BJU Int. 2013; 112:407–15.
Article25. Li H, Li C, Wu H, Zhang T, Wang J, Wang S, et al. Identification of Apo-A1 as a biomarker for early diagnosis of bladder transitional cell carcinoma. Proteome Sci. 2011; 9:21.
Article26. Zhang Z, Mao W, Wang L, Liu M, Zhang W, Wu Y, et al. Depletion of CDC5L inhibits bladder cancer tumorigenesis. J Cancer. 2020; 11:353–63.
Article27. Rose M, Gaisa NT, Antony P, Fiedler D, Heidenreich A, Otto W, et al. Epigenetic inactivation of ITIH5 promotes bladder cancer progression and predicts early relapse of pT1 high-grade urothelial tumours. Carcinogenesis. 2014; 35:727–36.
Article28. Chen YT, Chen HW, Domanski D, Smith DS, Liang KH, Wu CC, et al. Multiplexed quantification of 63 proteins in human urine by multiple reaction monitoring-based mass spectrometry for discovery of potential bladder cancer biomarkers. J Proteomics. 2012; 75:3529–45.
Article29. Gao X, Chen Y, Chen M, Wang S, Wen X, Zhang S. Identification of key candidate genes and biological pathways in bladder cancer. PeerJ. 2018; 6:e6036.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Urine Proteomics in the Era of Mass Spectrometry
- Advances in urinary biomarker discovery in urological research
- Proteomic profiling of bladder cancer for precision medicine in the clinical setting: A review for the busy urologist
- Biomarkers for Lung Cancer
- Comparative Examination on Bladder Washings and Urine for the Diagnosis of Bladder Cancer in Cytology