Nutr Res Pract.
2022 Jun;16(3):314-329. 10.4162/nrp.2022.16.3.314.
Reduction of oocyte lipid droplets and meiotic failure due to biotin deficiency was not rescued by restoring the biotin nutritional status
- Affiliations
-
- 1Department of Food Science and Nutrition, Faculty of Human Life and Environment, Nara Women's University, Nara 630-8506, Japan
- KMID: 2530393
- DOI: http://doi.org/10.4162/nrp.2022.16.3.314
Abstract
- BACKGROUND/OBJECTIVES
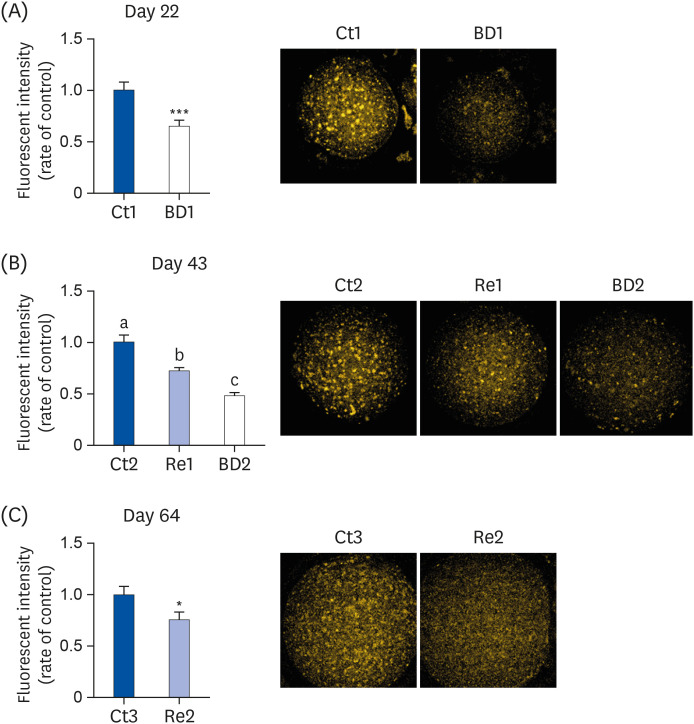
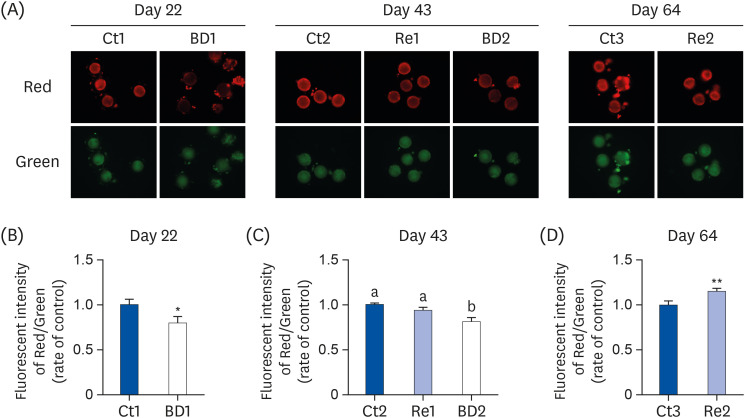
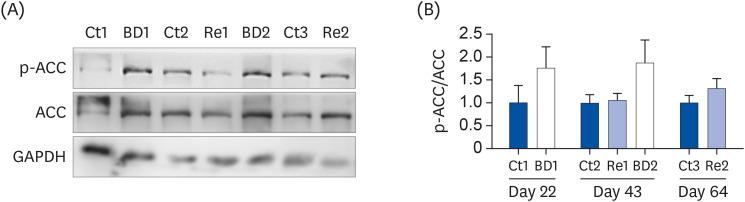
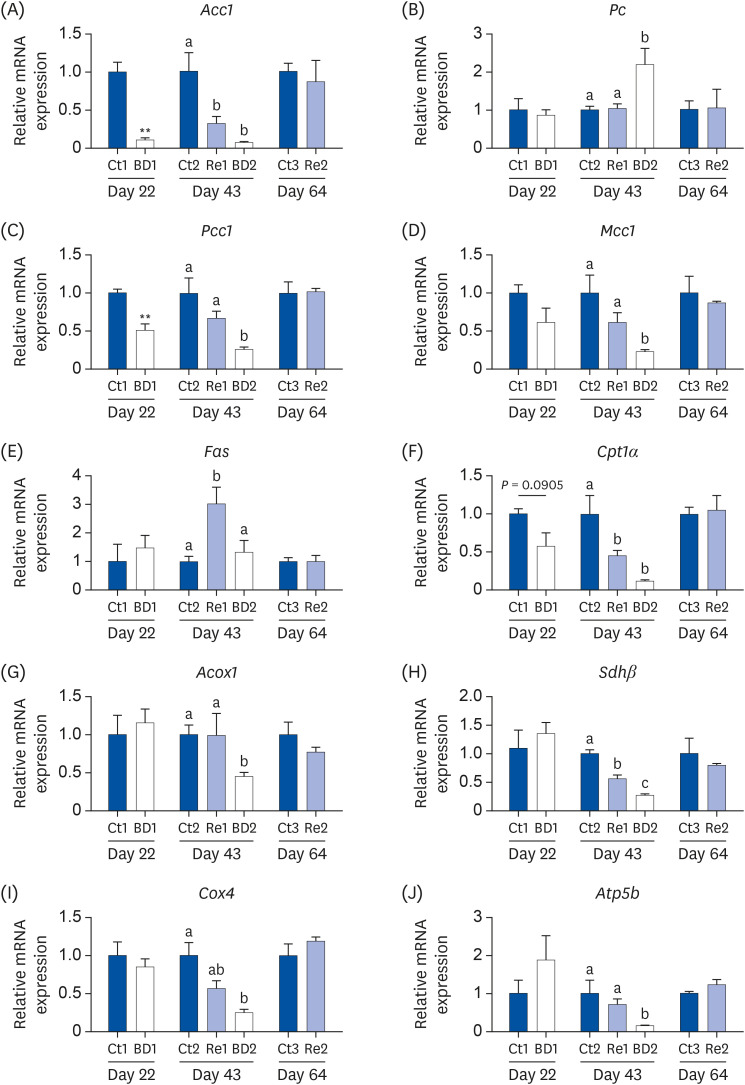
Oocyte lipid droplets play a crucial role in meiosis and embryo development. Biotin is associated with fatty acid synthesis and is the coenzyme for acetyl-CoA carboxylase (ACC). The effects of a biotin deficiency on the oocyte lipid metabolism remain unknown. This study examined the effects of a biotin deficiency and its replenishment on murine 1) oocyte lipid droplet levels, 2) ovary lipid metabolism, and 3) oocyte meiosis.
MATERIALS/METHODS
Mice were divided into 3 groups: control, biotin deficient (BD), and recovery groups. The control and BD groups were fed a control diet or BD diet (0.004 or 0 g biotin/kg), respectively. The recovery group mice were fed a BD diet until day 21, and were then fed the control diet from days 22 to 64. This study then quantified the oocyte lipid droplet levels, assessed the oocyte mitochondrial function, and examined the ability of oocytes to undergo meiosis. Ovarian phosphorylated ACC (p-ACC), lipogenesis, β-oxidation, and ATP production-related genes were evaluated.
RESULTS
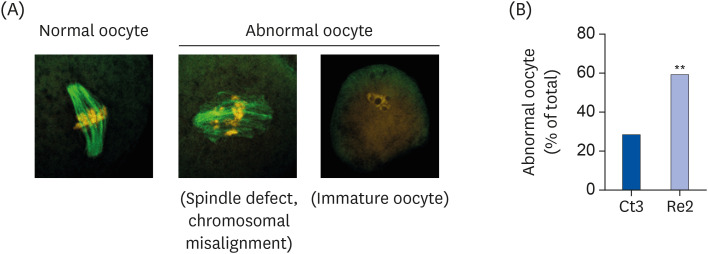
The BD group showed a decrease in lipid droplets and mitochondrial membrane potential and increased p-ACC levels. In the recovery group, the hepatic biotin concentration, ovarian p-ACC levels, and mitochondrial membrane potential were restored to the control group levels. On the other hand, the quantity of lipid droplets in the recovery group was not restored to the control levels. Furthermore, the percentage of oocytes with meiotic abnormalities was higher in the recovery group than in the control group.
CONCLUSIONS
A biotin deficiency reduced the oocyte lipid droplet levels by downregulating lipogenesis. The decreased lipid droplets and increased oocyte meiosis failure were not fully restored, even though the biotin nutrition status and gene expression of lipid metabolism was resumed. These results suggest that a biotin deficiency remains robust and can be longlasting. Biotin might play a crucial role in maintaining the oocyte quality.
Keyword
Figure
Reference
-
1. Fujimoto W, Inaoki M, Fukui T, Inoue Y, Kuhara T. Biotin deficiency in an infant fed with amino acid formula. J Dermatol. 2005; 32:256–261. PMID: 15863846.2. Fennelly J, Frank O, Baker H, Leevy CM. Peripheral neuropathy of the alcoholic: I, Aetiological role of aneurin and other B-complex vitamins. BMJ. 1964; 2:1290–1292. PMID: 14201210.3. Bonjour JP. Vitamins and alcoholism. V. Riboflavin, VI. Niacin, VII. Pantothenic acid, and VIII. Biotin. Int J Vitam Nutr Res. 1980; 50:425–440. PMID: 7009467.4. Subramanya SB, Subramanian VS, Kumar JS, Hoiness R, Said HM. Inhibition of intestinal biotin absorption by chronic alcohol feeding: cellular and molecular mechanisms. Am J Physiol Gastrointest Liver Physiol. 2011; 300:G494–G501. PMID: 21148397.5. Krause KH, Kochen W, Berlit P, Bonjour JP. Excretion of organic acids associated with biotin deficiency in chronic anticonvulsant therapy. Int J Vitam Nutr Res. 1984; 54:217–222. PMID: 6500847.6. Wolf B. Biotinidase deficiency and our champagne legacy. Gene. 2016; 589:142–150. PMID: 26456103.7. Tsuji A, Nakamura T, Shibata K. Biotin-deficient diet induces chromosome misalignment and spindle defects in mouse oocytes. Biosci Biotechnol Biochem. 2015; 79:292–299. PMID: 25301108.8. Valsangkar D, Downs SM. A requirement for fatty acid oxidation in the hormone-induced meiotic maturation of mouse oocytes. Biol Reprod. 2013; 89:43. PMID: 23863407.9. Tatsumi T, Takayama K, Ishii S, Yamamoto A, Hara T, Minami N, Miyasaka N, Kubota T, Matsuura A, Itakura E, et al. Forced lipophagy reveals that lipid droplets are required for early embryonic development in mouse. Development. 2018; 145:dev161893. PMID: 29475974.10. Algriany O, Vos PLAM, Sirard MA, Dieleman SJ. Switch in the expression of genes involved in lipid metabolism for in vivo-matured bovine oocytes and blastocysts. Reprod Fertil Dev. 2007; 19:244.11. Sanchez-Lazo L, Brisard D, Elis S, Maillard V, Uzbekov R, Labas V, Desmarchais A, Papillier P, Monget P, Uzbekova S. Fatty acid synthesis and oxidation in cumulus cells support oocyte maturation in bovine. Mol Endocrinol. 2014; 28:1502–1521. PMID: 25058602.12. Hassold T, Chiu D. Maternal age-specific rates of numerical chromosome abnormalities with special reference to trisomy. Hum Genet. 1985; 70:11–17. PMID: 3997148.13. Luzzo KM, Wang Q, Purcell SH, Chi M, Jimenez PT, Grindler N, Schedl T, Moley KH. High fat diet induced developmental defects in the mouse: oocyte meiotic aneuploidy and fetal growth retardation/brain defects. PLoS One. 2012; 7:e49217. PMID: 23152876.14. Hernández-Vázquez A, Ochoa-Ruiz E, Ibarra-González I, Ortega-Cuellar D, Salvador-Adriano A, Velázquez-Arellano A. Temporal development of genetic and metabolic effects of biotin deprivation. A search for the optimum time to study a vitamin deficiency. Mol Genet Metab. 2012; 107:345–351. PMID: 23010431.15. Solórzano-Vargas RS, Pacheco-Alvarez D, León-Del-Río A. Holocarboxylase synthetase is an obligate participant in biotin-mediated regulation of its own expression and of biotin-dependent carboxylases mRNA levels in human cells. Proc Natl Acad Sci U S A. 2002; 99:5325–5330. PMID: 11959985.16. Hernández-Vázquez A, Wolf B, Pindolia K, Ortega-Cuellar D, Hernández-González R, Heredia-Antúnez A, Ibarra-González I, Velázquez-Arellano A. Biotinidase knockout mice show cellular energy deficit and altered carbon metabolism gene expression similar to that of nutritional biotin deprivation: clues for the pathogenesis in the human inherited disorder. Mol Genet Metab. 2013; 110:248–254. PMID: 24075304.17. Suchy SF, Rizzo WB, Wolf B. Effect of biotin deficiency and supplementation on lipid metabolism in rats: saturated fatty acids. Am J Clin Nutr. 1986; 44:475–480. PMID: 3766434.18. Järvinen E, Ismail K, Muniandy M, Bogl LH, Heinonen S, Tummers M, Miettinen S, Kaprio J, Rissanen A, Ollikainen M, et al. Biotin-dependent functions in adiposity: a study of monozygotic twin pairs. Int J Obes. 2016; 40:788–795.19. Zhang X, Wu XQ, Lu S, Guo YL, Ma X. Deficit of mitochondria-derived ATP during oxidative stress impairs mouse MII oocyte spindles. Cell Res. 2006; 16:841–850. PMID: 16983401.20. Gorbsky GJ, Simerly C, Schatten G, Borisy GG. Microtubules in the metaphase-arrested mouse oocyte turn over rapidly. Proc Natl Acad Sci U S A. 1990; 87:6049–6053. PMID: 2385583.21. Bhuvaneswaran C, Dakshinamurti K. Energy-linked swelling of biotin-deficient rat liver mitochondria. Arch Biochem Biophys. 1971; 142:665–674. PMID: 4323730.22. Reeves PG. Components of the AIN-93 diets as improvements in the AIN-76A diet. J Nutr. 1997; 127:838S–841S. PMID: 9164249.23. Báez-Saldaña A, Camacho-Arroyo I, Espinosa-Aguirre JJ, Neri-Gómez T, Rojas-Ochoa A, Guerra-Araiza C, Larrieta E, Vital P, Díaz G, Chavira R, et al. Biotin deficiency and biotin excess: effects on the female reproductive system. Steroids. 2009; 74:863–869. PMID: 19540254.24. Liang S, Zhao MH, Ock SA, Kim NH, Cui XS. Fluoride impairs oocyte maturation and subsequent embryonic development in mice. Environ Toxicol. 2016; 31:1486–1495. PMID: 26011085.25. Genicot G, Leroy JL, Soom AV, Donnay I. The use of a fluorescent dye, Nile red, to evaluate the lipid content of single mammalian oocytes. Theriogenology. 2005; 63:1181–1194. PMID: 15710202.26. Tsuji A, Nakamura T, Shibata K. Effects of mild and severe vitamin b1 deficiencies on the meiotic maturation of mice oocytes. Nutr Metab Insights. 2017; 10:1178638817693824. PMID: 28469464.27. Fukui T, Iinuma K, Oizumi J, Izumi Y. Agar plate method using Lactobacillus plantarum for biotin determination in serum and urine. J Nutr Sci Vitaminol (Tokyo). 1994; 40:491–498. PMID: 7891209.28. Mock DM, Mock NI. Lymphocyte propionyl-CoA carboxylase is an early and sensitive indicator of biotin deficiency in rats, but urinary excretion of 3-hydroxypropionic acid is not. J Nutr. 2002; 132:1945–1950. PMID: 12097674.29. Atamna H, Newberry J, Erlitzki R, Schultz CS, Ames BN. Biotin deficiency inhibits heme synthesis and impairs mitochondria in human lung fibroblasts. J Nutr. 2007; 137:25–30. PMID: 17182796.30. Watanabe T, Endo A. Teratogenic effects of maternal biotin deficiency on mouse embryos examined at midgestation. Teratology. 1990; 42:295–300. PMID: 2274895.31. Watanabe T. Dietary biotin deficiency affects reproductive function and prenatal development in hamsters. J Nutr. 1993; 123:2101–2108. PMID: 8263603.32. Báez-Saldaña A, Gutiérrez-Ospina G, Chimal-Monroy J, Fernandez-Mejia C, Saavedra R. Biotin deficiency in mice is associated with decreased serum availability of insulin-like growth factor-I. Eur J Nutr. 2009; 48:137–144. PMID: 19165522.33. Báez-Saldaña A, Ortega E. Biotin deficiency blocks thymocyte maturation, accelerates thymus involution, and decreases nose-rump length in mice. J Nutr. 2004; 134:1970–1977. PMID: 15284385.34. Watanabe T. Teratogenic effects of biotin deficiency in mice. J Nutr. 1983; 113:574–581. PMID: 6827377.35. Halevy S, Diamant EJ, Guggenheim K. The effect of antibiotics on the metabolism of nicotinic acid, biotin and folic acid in rats. Br J Nutr. 1955; 9:57–62. PMID: 14351661.36. Chen L, Shu Y, Liang X, Chen EC, Yee SW, Zur AA, Li S, Xu L, Keshari KR, Lin MJ, et al. OCT1 is a high-capacity thiamine transporter that regulates hepatic steatosis and is a target of metformin. Proc Natl Acad Sci U S A. 2014; 111:9983–9988. PMID: 24961373.37. Schlaepfer IR, Joshi M. CPT1A-mediated fat oxidation, mechanisms, and therapeutic potential. Endocrinology. 2020; 161:bqz046. PMID: 31900483.38. Regueira M, Riera MF, Galardo MN, Camberos MC, Pellizzari EH, Cigorraga SB, Meroni SB. FSH and bFGF regulate the expression of genes involved in Sertoli cell energetic metabolism. Gen Comp Endocrinol. 2015; 222:124–133. PMID: 26315388.39. Osada K, Komai M, Sugiyama K, Urayama N, Furukawa Y. Experimental study of fatigue provoked by biotin deficiency in mice. Int J Vitam Nutr Res. 2004; 74:334–340. PMID: 15628671.40. Zhu Y, Liu G, Du X, Shi Z, Jin M, Sha X, Li X, Wang Z, Li X. Expression patterns of hepatic genes involved in lipid metabolism in cows with subclinical or clinical ketosis. J Dairy Sci. 2019; 102:1725–1735. PMID: 30471902.41. Downs SM, Mosey JL, Klinger J. Fatty acid oxidation and meiotic resumption in mouse oocytes. Mol Reprod Dev. 2009; 76:844–853. PMID: 19455666.42. Selvam S, Ramaian Santhaseela A, Ganesan D, Rajasekaran S, Jayavelu T. Autophagy inhibition by biotin elicits endoplasmic reticulum stress to differentially regulate adipocyte lipid and protein synthesis. Cell Stress Chaperones. 2019; 24:343–350. PMID: 30648232.43. Cordonier EL, Jarecke SK, Hollinger FE, Zempleni J. Inhibition of acetyl-CoA carboxylases by soraphen A prevents lipid accumulation and adipocyte differentiation in 3T3-L1 cells. Eur J Pharmacol. 2016; 780:202–208. PMID: 27041646.44. Ochoa-Ruiz E, Díaz-Ruiz R, Hernández-Vázquez AJ, Ibarra-González I, Ortiz-Plata A, Rembao D, Ortega-Cuéllar D, Viollet B, Uribe-Carvajal S, Corella JA, et al. Biotin deprivation impairs mitochondrial structure and function and has implications for inherited metabolic disorders. Mol Genet Metab. 2015; 116:204–214. PMID: 26343941.45. Hou YJ, Zhu CC, Duan X, Liu HL, Wang Q, Sun SC. Both diet and gene mutation induced obesity affect oocyte quality in mice. Sci Rep. 2016; 6:18858. PMID: 26732298.46. Reynolds KA, Boudoures AL, Chi MM, Wang Q, Moley KH. Adverse effects of obesity and/or high-fat diet on oocyte quality and metabolism are not reversible with resumption of regular diet in mice. Reprod Fertil Dev. 2015; 27:716–724. PMID: 25775080.47. Robenek H, Hofnagel O, Buers I, Robenek MJ, Troyer D, Severs NJ. Adipophilin-enriched domains in the ER membrane are sites of lipid droplet biogenesis. J Cell Sci. 2006; 119:4215–4224. PMID: 16984971.48. Gong J, Sun Z, Wu L, Xu W, Schieber N, Xu D, Shui G, Yang H, Parton RG, Li P. Fsp27 promotes lipid droplet growth by lipid exchange and transfer at lipid droplet contact sites. J Cell Biol. 2011; 195:953–963. PMID: 22144693.49. Li Y, Na K, Lee HJ, Lee EY, Paik YK. Contribution of sams-1 and pmt-1 to lipid homoeostasis in adult Caenorhabditis elegans. J Biochem. 2011; 149:529–538. PMID: 21389045.50. Cohen BC, Shamay A, Argov-Argaman N. Regulation of lipid droplet size in mammary epithelial cells by remodeling of membrane lipid composition-a potential mechanism. PLoS One. 2015; 10:e0121645. PMID: 25756421.51. Camporeale G, Zempleni J, Eissenberg JC. Susceptibility to heat stress and aberrant gene expression patterns in holocarboxylase synthetase-deficient Drosophila melanogaster are caused by decreased biotinylation of histones, not of carboxylases. J Nutr. 2007; 137:885–889. PMID: 17374649.52. Kothapalli N, Sarath G, Zempleni J. Biotinylation of K12 in histone H4 decreases in response to DNA double-strand breaks in human JAr choriocarcinoma cells. J Nutr. 2005; 135:2337–2342. PMID: 16177192.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Serum Biotin Levels in General Korean Population
- Comparison of Biotin Interference in Second- and Third-Generation Roche Free Thyroxine Immunoassays
- Urea Synthesis in the Intact and in the Isolated Perfused Liver of the Biotin-Deficient Rats
- Spurious Thyroid Function Test Results due to Biotin Interference: a Report of Three Cases and a Literature Review
- A Case of Lennox-Gastaut Syndrome due to 3-Methylcrotonyl CoA Carboxylase Deficiency