Nutr Res Pract.
2022 Jun;16(3):285-297. 10.4162/nrp.2022.16.3.285.
Dietary supplementation with Korean pine nut oil decreases body fat accumulation and dysregulation of the appetite-suppressing pathway in the hypothalamus of high-fat diet-induced obese mice
- Affiliations
-
- 1Major of Food and Nutrition, Division of Applied Food System, Seoul Women's University, Seoul 01797, Korea
- 2Department of Food and Nutrition, College of Human Ecology, Seoul National University, Seoul 08826, Korea
- 3Research Institute of Human Ecology, Seoul National University, Seoul 08826, Korea
- KMID: 2530391
- DOI: http://doi.org/10.4162/nrp.2022.16.3.285
Abstract
- BACKGROUND/OBJECTIVES
Korean pine nut oil (PNO) has been reported to suppress appetite by increasing satiety hormone release. However, previous studies have rendered inconsistent results and there is lack of information on whether dietary Korean PNO affects the expression of satiety hormone receptors and hypothalamic neuropeptides. Therefore, our study sought to evaluate the chronic effects of Korean PNO on the long-term regulation of energy balance.
MATERIALS/METHODS
Five-week-old male C57BL/6 mice were fed with control diets containing 10% kcal fat from Korean PNO or soybean oil (SBO) (PC or SC) or high-fat diets (HFDs) containing 35% kcal fat from lard and 10% kcal fat from Korean PNO or SBO (PHFD or SHFD) for 12 weeks. The expression of gastrointestinal satiety hormone receptors, hypothalamic neuropeptides, and genes related to intestinal lipid absorption and adipose lipid metabolism was then measured.
RESULTS
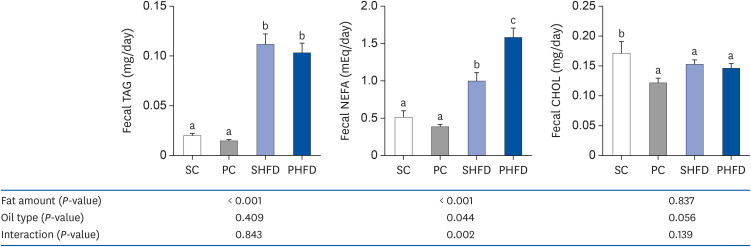
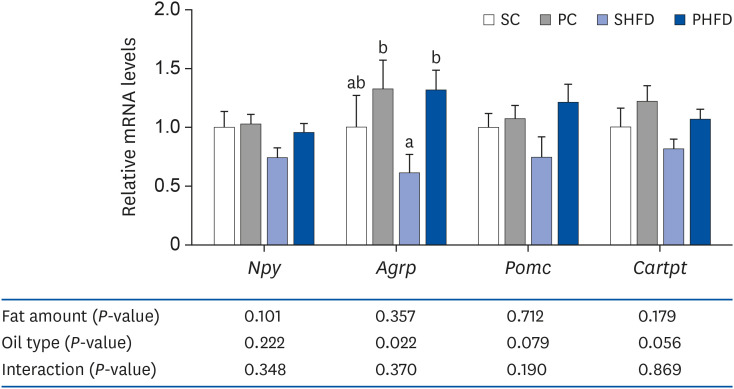
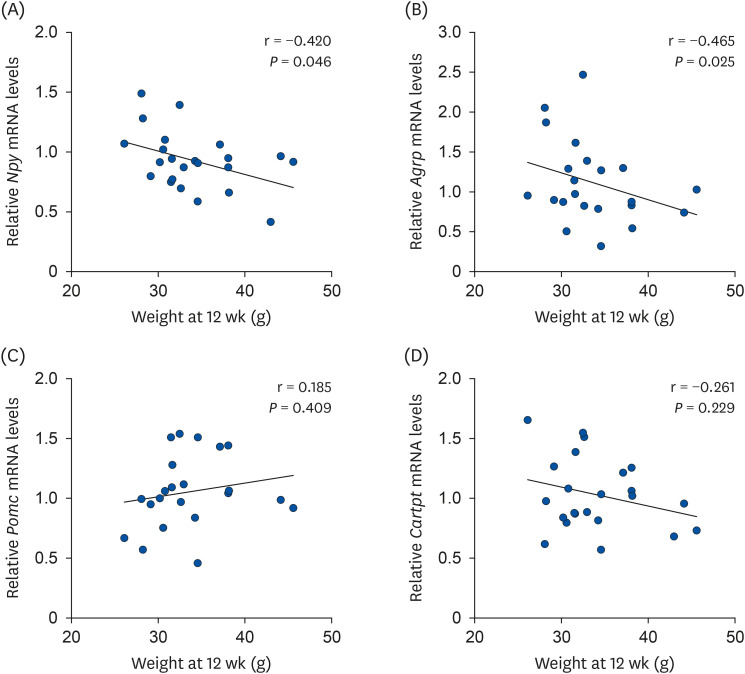
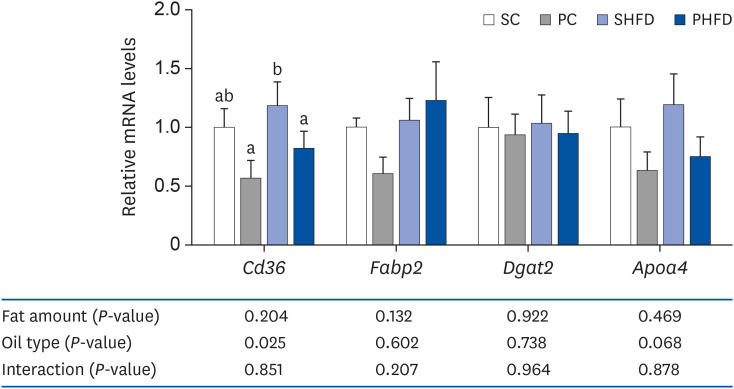
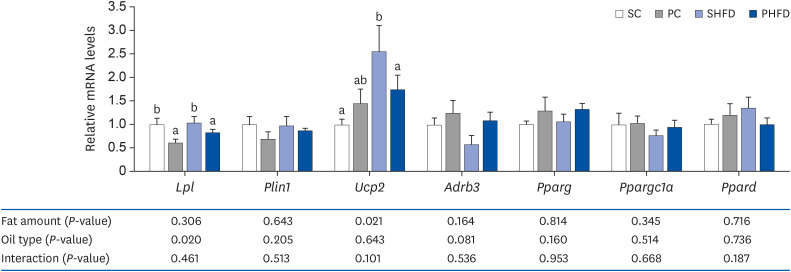
There was no difference in the daily food intake between PNO- and SBO-fed mice; however, the PC and PHFD groups accumulated 30% and 18% less fat compared to SC and SHFD, respectively. Korean PNO-fed mice exhibited higher messenger RNA (mRNA) expression of Ghsr (ghrelin receptor) and ,Agrp (agouti-related peptide) (P < 0.05), which are expressed when energy consumption is low to induce appetite as well as the appetitesuppressing neuropeptides Pomc and Cartpt (P = 0.079 and 0.056, respectively). Korean PNO downregulated jejunal Cd36 and epididymal Lpl mRNA expressions, which could suppress intestinal fatty acid absorption and fat storage in white adipose tissue. Consistent with these findings, Korean PNO-fed mice had higher levels of fecal non-esterified fatty acid excretion. Korean PNO also tended to downregulate jejunal Apoa4 and upregulate epididymal Adrb3 mRNA levels, suggesting that PNO may decrease chylomicron synthesis and induce lipolysis.
CONCLUSIONS
In summary, Korean PNO attenuated body fat accumulation, and appeared to prevent HFD-induced dysregulation of the hypothalamic appetite-suppressing pathway.
Keyword
Figure
Reference
-
1. Badman MK, Flier JS. The gut and energy balance: visceral allies in the obesity wars. Science. 2005; 307:1909–1914. PMID: 15790843.2. Sainsbury A, Cooney GJ, Herzog H. Hypothalamic regulation of energy homeostasis. Best Pract Res Clin Endocrinol Metab. 2002; 16:623–637. PMID: 12468411.3. Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA. 1999; 282:1523–1529. PMID: 10546691.4. Wolff RL, Pédrono F, Pasquier E, Marpeau AM. General characteristics of Pinus spp. seed fatty acid compositions, and importance of delta5-olefinic acids in the taxonomy and phylogeny of the genus. Lipids. 2000; 35:1–22. PMID: 10695919.5. Lee JW, Lee KW, Lee SW, Kim IH, Rhee C. Selective increase in pinolenic acid (all-cis-5,9,12-18:3) in Korean pine nut oil by crystallization and its effect on LDL-receptor activity. Lipids. 2004; 39:383–387. PMID: 15357026.6. Matsuo N, Osada K, Kodama T, Lim BO, Nakao A, Yamada K, Sugano M. Effects of γ-linolenic acid and its positional isomer pinolenic acid on immune parameters of brown-Norway rats. Prostaglandins Leukot Essent Fatty Acids. 1996; 55:223–229. PMID: 8951990.7. Pasman WJ, Heimerikx J, Rubingh CM, van den Berg R, O'Shea M, Gambelli L, Hendriks HF, Einerhand AW, Scott C, Keizer HG, et al. The effect of Korean pine nut oil on in vitro CCK release, on appetite sensations and on gut hormones in post-menopausal overweight women. Lipids Health Dis. 2008; 7:10. PMID: 18355411.8. Zhu S, Park S, Lim Y, Shin S, Han SN. Korean pine nut oil replacement decreases intestinal lipid uptake while improves hepatic lipid metabolism in mice. Nutr Res Pract. 2016; 10:477–486. PMID: 27698954.9. Le NH, Shin S, Tu TH, Kim CS, Kang JH, Tsuyoshi G, Teruo K, Han SN, Yu R. Diet enriched with Korean pine nut oil improves mitochondrial oxidative metabolism in skeletal muscle and brown adipose tissue in diet-induced obesity. J Agric Food Chem. 2012; 60:11935–11941. PMID: 23140571.10. Park S, Shin S, Lim Y, Shin JH, Seong JK, Han SN. Korean pine nut oil attenuated hepatic triacylglycerol accumulation in high-fat diet-induced obese mice. Nutrients. 2016; 8:59.11. Sugano M, Ikeda I, Wakamatsu K, Oka T. Influence of Korean pine (Pinus koraiensis)-seed oil containing cis-5,cis-9,cis-12-octadecatrienoic acid on polyunsaturated fatty acid metabolism, eicosanoid production and blood pressure of rats. Br J Nutr. 1994; 72:775–783. PMID: 7826999.12. Hughes GM, Boyland EJ, Williams NJ, Mennen L, Scott C, Kirkham TC, Harrold JA, Keizer HG, Halford JC. The effect of Korean pine nut oil (PinnoThin) on food intake, feeding behaviour and appetite: a double-blind placebo-controlled trial. Lipids Health Dis. 2008; 7:6. PMID: 18307772.13. Verhoef SP, Westerterp KR. No effects of Korean pine nut triacylglycerol on satiety and energy intake. Nutr Metab (Lond). 2011; 8:79. PMID: 22074178.14. Park S, Lim Y, Shin S, Han SN. Impact of Korean pine nut oil on weight gain and immune responses in high-fat diet-induced obese mice. Nutr Res Pract. 2013; 7:352–358. PMID: 24133613.15. Cummings DE, Overduin J. Gastrointestinal regulation of food intake. J Clin Invest. 2007; 117:13–23. PMID: 17200702.16. Morash MG, Gagnon J, Nelson S, Anini Y. Tissue distribution and effects of fasting and obesity on the ghrelin axis in mice. Regul Pept. 2010; 163:62–73. PMID: 20362013.17. Moesgaard SG, Ahrén B, Carr RD, Gram DX, Brand CL, Sundler F. Effects of high-fat feeding and fasting on ghrelin expression in the mouse stomach. Regul Pept. 2004; 120:261–267. PMID: 15177945.18. Holst B, Schwartz TW. Constitutive ghrelin receptor activity as a signaling set-point in appetite regulation. Trends Pharmacol Sci. 2004; 25:113–117. PMID: 15058279.19. la Fleur SE, van Rozen AJ, Luijendijk MC, Groeneweg F, Adan RA. A free-choice high-fat high-sugar diet induces changes in arcuate neuropeptide expression that support hyperphagia. Int J Obes. 2010; 34:537–546.20. Cifani C, Micioni Di Bonaventura MV, Pucci M, Giusepponi ME, Romano A, Di Francesco A, Maccarrone M, D'Addario C. Regulation of hypothalamic neuropeptides gene expression in diet induced obesity resistant rats: possible targets for obesity prediction? Front Neurosci. 2015; 9:187. PMID: 26106286.21. Lin S, Storlien LH, Huang XF. Leptin receptor, NPY, POMC mRNA expression in the diet-induced obese mouse brain. Brain Res. 2000; 875:89–95. PMID: 10967302.22. Drover VA, Ajmal M, Nassir F, Davidson NO, Nauli AM, Sahoo D, Tso P, Abumrad NA. CD36 deficiency impairs intestinal lipid secretion and clearance of chylomicrons from the blood. J Clin Invest. 2005; 115:1290–1297. PMID: 15841205.23. Simon T, Cook VR, Rao A, Weinberg RB. Impact of murine intestinal apolipoprotein A-IV expression on regional lipid absorption, gene expression, and growth. J Lipid Res. 2011; 52:1984–1994. PMID: 21840868.24. Mead JR, Irvine SA, Ramji DP. Lipoprotein lipase: structure, function, regulation, and role in disease. J Mol Med (Berl). 2002; 80:753–769. PMID: 12483461.25. Peirce V, Carobbio S, Vidal-Puig A. The different shades of fat. Nature. 2014; 510:76–83. PMID: 24899307.26. Shin S, Ajuwon KM. Divergent response of murine and porcine adipocytes to stimulation of browning genes by 18-carbon polyunsaturated fatty acids and beta-receptor agonists. Lipids. 2018; 53:65–75. PMID: 29424439.27. Rippe C, Berger K, Böiers C, Ricquier D, Erlanson-Albertsson C. Effect of high-fat diet, surrounding temperature, and enterostatin on uncoupling protein gene expression. Am J Physiol Endocrinol Metab. 2000; 279:E293–E300. PMID: 10913028.28. Surwit RS, Wang S, Petro AE, Sanchis D, Raimbault S, Ricquier D, Collins S. Diet-induced changes in uncoupling proteins in obesity-prone and obesity-resistant strains of mice. Proc Natl Acad Sci U S A. 1998; 95:4061–4065. PMID: 9520493.29. Chen M, Yang YK, Loux TJ, Georgeson KE, Harmon CM. The role of hyperglycemia in FAT/CD36 expression and function. Pediatr Surg Int. 2006; 22:647–654. PMID: 16838191.30. Hanniman EA, Lambert G, Inoue Y, Gonzalez FJ, Sinal CJ. Apolipoprotein A-IV is regulated by nutritional and metabolic stress: involvement of glucocorticoids, HNF-4 alpha, and PGC-1 alpha. J Lipid Res. 2006; 47:2503–2514. PMID: 16929032.31. Dewulf EM, Cani PD, Neyrinck AM, Possemiers S, Van Holle A, Muccioli GG, Deldicque L, Bindels LB, Pachikian BD, Sohet FM, et al. Inulin-type fructans with prebiotic properties counteract GPR43 overexpression and PPARγ-related adipogenesis in the white adipose tissue of high-fat diet-fed mice. J Nutr Biochem. 2011; 22:712–722. PMID: 21115338.32. Shin S, Ajuwon KM. Effects of diets differing in composition of 18-C fatty acids on adipose tissue thermogenic gene expression in mice fed high-fat diets. Nutrients. 2018; 10:E256. PMID: 29473916.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Impact of Korean pine nut oil on weight gain and immune responses in high-fat diet-induced obese mice
- Effect of Korean pine nut oil on hepatic iron, copper, and zinc status and expression of genes and proteins related to iron absorption in dietinduced obese mice
- Effects of Calcium and Genistein on Body Fat and Lipid Metabolism in High Fat-induced Obese Mice
- Korean pine nut oil replacement decreases intestinal lipid uptake while improves hepatic lipid metabolism in mice
- Effects of D-allulose on body fat accumulation in rats fed severely carbohydrate-restricted diets containing beef tallow or soybean oil