Nutr Res Pract.
2016 Oct;10(5):477-486. 10.4162/nrp.2016.10.5.477.
Korean pine nut oil replacement decreases intestinal lipid uptake while improves hepatic lipid metabolism in mice
- Affiliations
-
- 1Department of Food and Nutrition, College of Human Ecology, Seoul National University, 1 Gwanak-ro, Gwanak-gu, Seoul 08826, Korea. snhan@snu.ac.kr
- 2Research Institute of Human Ecology, Seoul National University, Seoul 08826, Korea.
- KMID: 2434081
- DOI: http://doi.org/10.4162/nrp.2016.10.5.477
Abstract
- BACKGROUND/OBJECTIVES
Consumption of pine nut oil (PNO) was shown to reduce weight gain and attenuate hepatic steatosis in mice fed a high-fat diet (HFD). The aim of this study was to examine the effects of PNO on both intestinal and hepatic lipid metabolism in mice fed control or HFD.
MATERIALS/METHODS
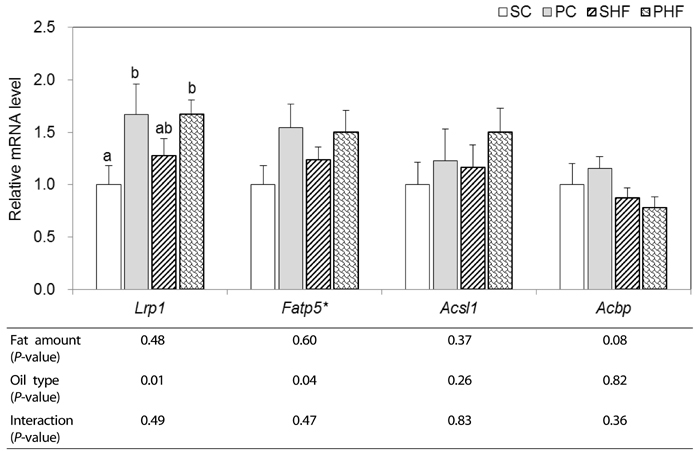
Five-week-old C57BL/6 mice were fed control diets containing 10% energy fat from either Soybean Oil (SBO) or PNO, or HFD containing 15% energy fat from lard and 30% energy fat from SBO or PNO for 12 weeks. Expression of genes related to intestinal fatty acid (FA) uptake and channeling (Cd36, Fatp4, Acsl5, Acbp), intestinal chylomicron synthesis (Mtp, ApoB48, ApoA4), hepatic lipid uptake and channeling (Lrp1, Fatp5, Acsl1, Acbp), hepatic triacylglycerol (TAG) lipolysis and FA oxidation (Atgl, Cpt1a, Acadl, Ehhadh, Acaa1), as well as very low-density lipoprotein (VLDL) assembly (ApoB100) were determined by real-time PCR.
RESULTS
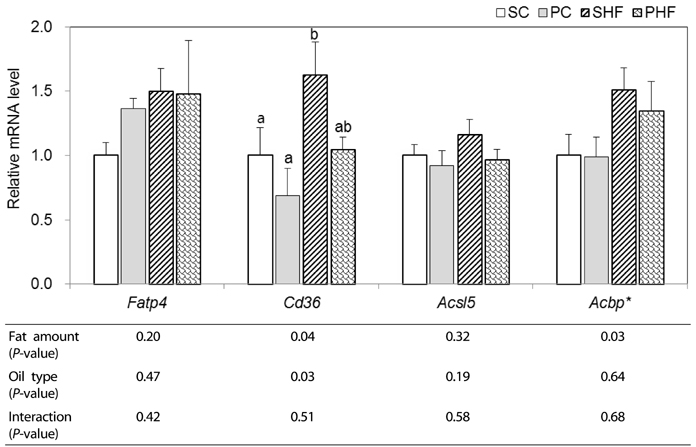
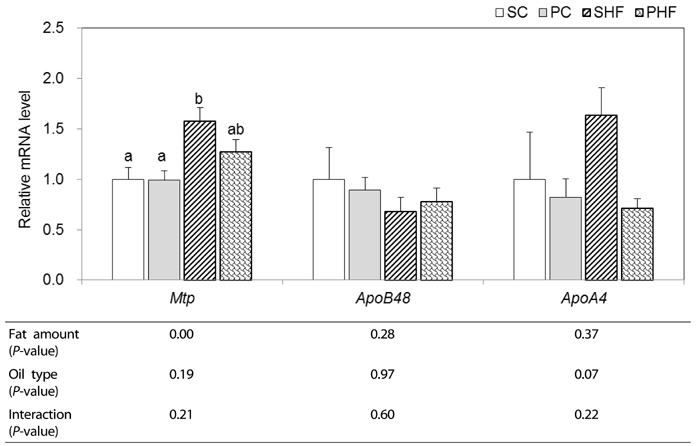
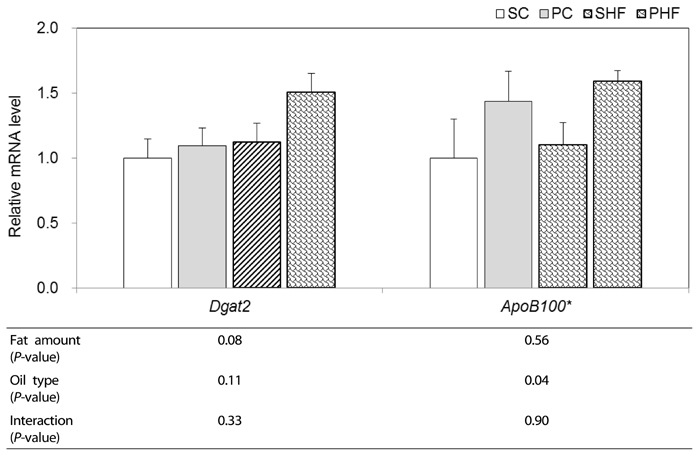
In intestine, significantly lower Cd36 mRNA expression (P < 0.05) and a tendency of lower ApoA4 mRNA levels (P = 0.07) was observed in PNO-fed mice, indicating that PNO consumption may decrease intestinal FA uptake and chylomicron assembly. PNO consumption tended to result in higher hepatic mRNA levels of Atgl (P = 0.08) and Cpt1a (P = 0.05). Significantly higher hepatic mRNA levels of Acadl and ApoB100 were detected in mice fed PNO diet (P < 0.05). These results suggest that PNO could increase hepatic TAG metabolism; mitochondrial fatty acid oxidation and VLDL assembly.
CONCLUSIONS
PNO replacement in the diet might function in prevention of excessive lipid uptake by intestine and improve hepatic lipid metabolism in both control diet and HFD fed mice.
Keyword
MeSH Terms
Figure
Reference
-
1. de Wit N, Derrien M, Bosch-Vermeulen H, Oosterink E, Keshtkar S, Duval C, de Vogel-van den Bosch J, Kleerebezem M, Müller M, van der Meer R. Saturated fat stimulates obesity and hepatic steatosis and affects gut microbiota composition by an enhanced overflow of dietary fat to the distal intestine. Am J Physiol Gastrointest Liver Physiol. 2012; 303:G589–G599.
Article2. Abumrad NA, Davidson NO. Role of the gut in lipid homeostasis. Physiol Rev. 2012; 92:1061–1085.
Article3. Nguyen P, Leray V, Diez M, Serisier S, Le Bloc'h J, Siliart B, Dumon H. Liver lipid metabolism. J Anim Physiol Anim Nutr (Berl). 2008; 92:272–283.
Article4. Kim S, Sohn I, Ahn JI, Lee KH, Lee YS, Lee YS. Hepatic gene expression profiles in a long-term high-fat diet-induced obesity mouse model. Gene. 2004; 340:99–109.
Article5. Fabbrini E, Sullivan S, Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology. 2010; 51:679–689.
Article6. Turpin SM, Hoy AJ, Brown RD, Rudaz CG, Honeyman J, Matzaris M, Watt MJ. Adipose triacylglycerol lipase is a major regulator of hepatic lipid metabolism but not insulin sensitivity in mice. Diabetologia. 2011; 54:146–156.
Article7. Green CJ, Hodson L. The influence of dietary fat on liver fat accumulation. Nutrients. 2014; 6:5018–5033.
Article8. Lee JW, Lee KW, Lee SW, Kim IH, Rhee C. Selective increase in pinolenic acid (all-cis-5,9,12-18:3) in Korean pine nut oil by crystallization and its effect on LDL-receptor activity. Lipids. 2004; 39:383–387.
Article9. Ferramosca A, Savy V, Einerhand AW, Zara V. Pinus koraiensis seed oil (PinnoThinTM) supplementation reduces body weight gain and lipid concentration in liver and plasma of mice. J Anim Feed Sci. 2008; 17:621–630.
Article10. Asset G, Staels B, Wolff RL, Baugé E, Madj Z, Fruchart JC, Dallongeville J. Effects of Pinus pinaster and Pinus koraiensis seed oil supplementation on lipoprotein metabolism in the rat. Lipids. 1999; 34:39–44.
Article11. Park S, Lim Y, Shin S, Han SN. Impact of Korean pine nut oil on weight gain and immune responses in high-fat diet-induced obese mice. Nutr Res Pract. 2013; 7:352–358.
Article12. Le NH, Shin S, Tu TH, Kim CS, Kang JH, Tsuyoshi G, Teruo K, Han SN, Yu R. Diet enriched with Korean pine nut oil improves mitochondrial oxidative metabolism in skeletal muscle and brown adipose tissue in diet-induced obesity. J Agric Food Chem. 2012; 60:11935–11941.
Article13. Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957; 226:497–509.
Article14. Petit V, Arnould L, Martin P, Monnot MC, Pineau T, Besnard P, Niot I. Chronic high-fat diet affects intestinal fat absorption and postprandial triglyceride levels in the mouse. J Lipid Res. 2007; 48:278–287.
Article15. Huang W, Liu R, Ou Y, Li X, Qiang O, Yu T, Tang CW. Octreotide promotes weight loss via suppression of intestinal MTP and apoB48 expression in diet-induced obesity rats. Nutrition. 2013; 29:1259–1265.
Article16. Iqbal J, Hussain MM. Intestinal lipid absorption. Am J Physiol Endocrinol Metab. 2009; 296:E1183–E1194.
Article17. Black DD. Development and physiological regulation of intestinal lipid absorption. I. Development of intestinal lipid absorption: cellular events in chylomicron assembly and secretion. Am J Physiol Gastrointest Liver Physiol. 2007; 293:G519–G524.
Article18. Lu S, Yao Y, Cheng X, Mitchell S, Leng S, Meng S, Gallagher JW, Shelness GS, Morris GS, Mahan J, Frase S, Mansbach CM, Weinberg RB, Black DD. Overexpression of apolipoprotein A-IV enhances lipid secretion in IPEC-1 cells by increasing chylomicron size. J Biol Chem. 2006; 281:3473–3483.
Article19. Stan S, Delvin E, Lambert M, Seidman E, Levy E. Apo A-IV: an update on regulation and physiologic functions. Biochim Biophys Acta. 2003; 1631:177–187.
Article20. Mortimer BC, Beveridge DJ, Martins IJ, Redgrave TG. Intracellular localization and metabolism of chylomicron remnants in the livers of low density lipoprotein receptor-deficient mice and apoEdeficient mice. Evidence for slow metabolism via an alternative apoE-dependent pathway. J Biol Chem. 1995; 270:28767–28776.
Article21. Nestel PJ, Havel RJ, Bezman A. Sites of initial removal of chylomicron triglyceride fatty acids from the blood. J Clin Invest. 1962; 41:1915–1921.
Article22. Lillis AP, Van Duyn LB, Murphy-Ullrich JE, Strickland DK. LDL receptor-related protein 1: unique tissue-specific functions revealed by selective gene knockout studies. Physiol Rev. 2008; 88:887–918.
Article23. Masson O, Chavey C, Dray C, Meulle A, Daviaud D, Quilliot D, Muller C, Valet P, Liaudet-Coopman E. LRP1 receptor controls adipogenesis and is up-regulated in human and mouse obese adipose tissue. PLoS One. 2009; 4:e7422.
Article24. Willnow TE. Mechanisms of hepatic chylomicron remnant clearance. Diabet Med. 1997; 14:Suppl 3. S75–S80.
Article25. Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005; 115:1343–1351.
Article26. Hardwick JP, Osei-Hyiaman D, Wiland H, Abdelmegeed MA, Song BJ. PPAR/RXR regulation of fatty acid metabolism and fatty acid omega-hydroxylase (CYP4) isozymes: implications for prevention of lipotoxicity in fatty liver disease. PPAR Res. 2009; 2009:952734.27. Adiels M, Olofsson SO, Taskinen MR, Borén J. Overproduction of very low-density lipoproteins is the hallmark of the dyslipidemia in the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2008; 28:1225–1236.
Article28. Grenier-Larouche T, Labbé SM, Noll C, Richard D, Carpentier AC. Metabolic inflexibility of white and brown adipose tissues in abnormal fatty acid partitioning of type 2 diabetes. Int J Obes Suppl. 2012; 2:S37–S42.
Article29. Bjørndal B, Burri L, Staalesen V, Skorve J, Berge RK. Different adipose depots: their role in the development of metabolic syndrome and mitochondrial response to hypolipidemic agents. J Obes. 2011; 2011:490650.
Article30. Ong KT, Mashek MT, Bu SY, Greenberg AS, Mashek DG. Adipose triglyceride lipase is a major hepatic lipase that regulates triacylglycerol turnover and fatty acid signaling and partitioning. Hepatology. 2011; 53:116–126.
Article31. Guo Y, Jolly RA, Halstead BW, Baker TK, Stutz JP, Huffman M, Calley JN, West A, Gao H, Searfoss GH, Li S, Irizarry AR, Qian HR, Stevens JL, Ryan TP. Underlying mechanisms of pharmacology and toxicity of a novel PPAR agonist revealed using rodent and canine hepatocytes. Toxicol Sci. 2007; 96:294–309.
Article32. van der, Bloks VW, Grefhorst A, Hoekstra J, Gerding A, Kooi K, Gerbens F, te Meerman G, Kuipers F. Gene expression profiling in livers of mice after acute inhibition of beta-oxidation. Genomics. 2007; 90:680–689.
Article33. Millar JS, Stone SJ, Tietge UJ, Tow B, Billheimer JT, Wong JS, Hamilton RL, Farese RV Jr, Rader DJ. Short-term overexpression of DGAT1 or DGAT2 increases hepatic triglyceride but not VLDL triglyceride or apoB production. J Lipid Res. 2006; 47:2297–2305.
Article34. Miccoli R, Bianchi C, Penno G, Del Prato S. Insulin resistance and lipid disorders. Future Lipidol. 2008; 3:651–664.
Article35. Heath RB, Karpe F, Milne RW, Burdge GC, Wootton SA, Frayn KN. Selective partitioning of dietary fatty acids into the VLDL TG pool in the early postprandial period. J Lipid Res. 2003; 44:2065–2072.
Article36. Geerling JJ, Boon MR, van der, van den, van den, Lombès M, Princen HM, Havekes LM, Rensen PC, Guigas B. Metformin lowers plasma triglycerides by promoting VLDLtriglyceride clearance by brown adipose tissue in mice. Diabetes. 2014; 63:880–891.
Article37. Cooper AD. Hepatic uptake of chylomicron remnants. J Lipid Res. 1997; 38:2173–2192.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Impact of Korean pine nut oil on weight gain and immune responses in high-fat diet-induced obese mice
- The Effect of Rice Germ Oil Supplement on Serum and Hepatic Lipid Levels of Streptozotocin-Induced Diabetic Mice
- Dietary supplementation with Korean pine nut oil decreases body fat accumulation and dysregulation of the appetite-suppressing pathway in the hypothalamus of high-fat diet-induced obese mice
- Supplementary Effect of gamma-Oryzanol on Lipid Metabolism in Diabetic KK Mice
- Effect of Fish Oil Diet on Activities of Lipogenic Enzymes and Glucose -6 -phosphatase in Rat Liver and Adipose