Ann Surg Treat Res.
2022 Jun;102(6):335-341. 10.4174/astr.2022.102.6.335.
Diagnostic and prognostic impact of fluorodeoxyglucosepositron emission tomography in diagnosing intraductal papillary neoplasms of the bile duct of the liver
- Affiliations
-
- 1Department of Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 2Department of Nuclear Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 3Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2530388
- DOI: http://doi.org/10.4174/astr.2022.102.6.335
Abstract
- Purpose
Malignant intraductal papillary neoplasm of the bile duct of the liver (IPNB-L) cannot readily be diagnosed through preoperative CT or MRI, but fluorodeoxyglucose (FDG)-PET is a viable alternative. This study evaluated the diagnostic and prognostic impacts of FDG-PET in patients with IPNB-L.
Methods
This was a retrospective single-center study of 101 IPNB-L patients who underwent hepatectomy between 2010 and 2019.
Results
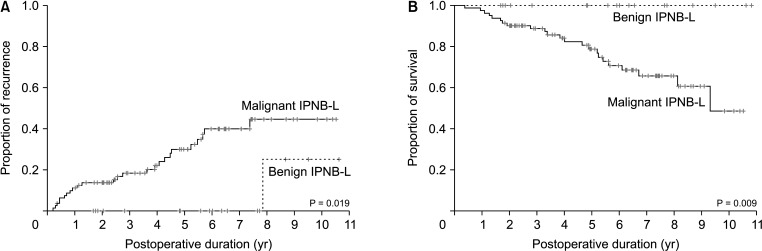
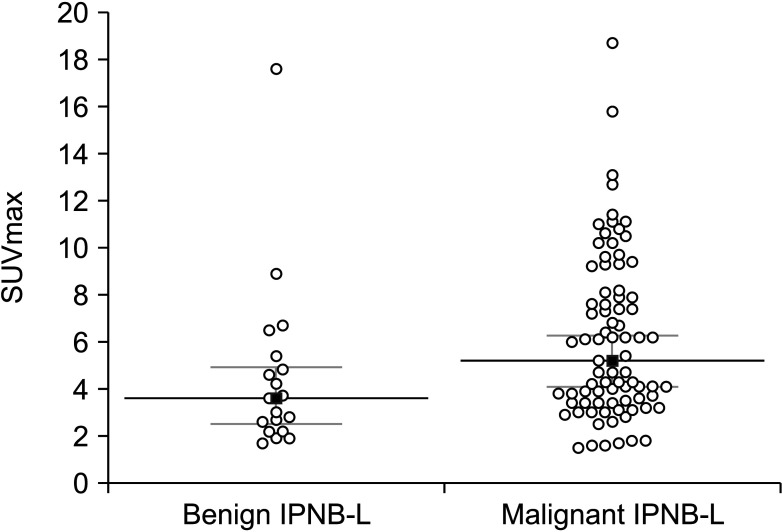
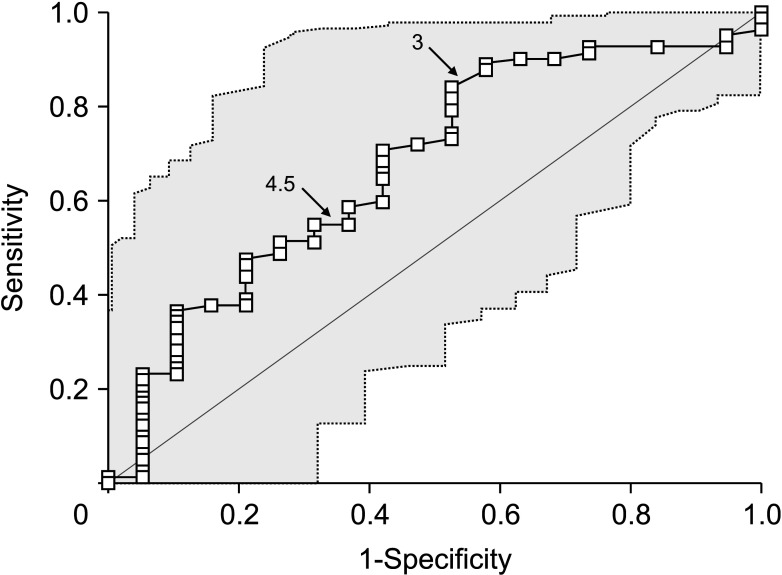
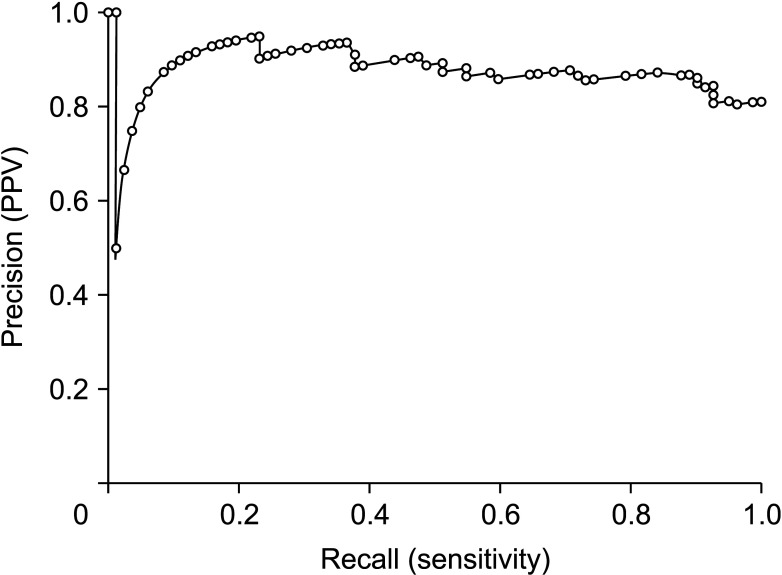
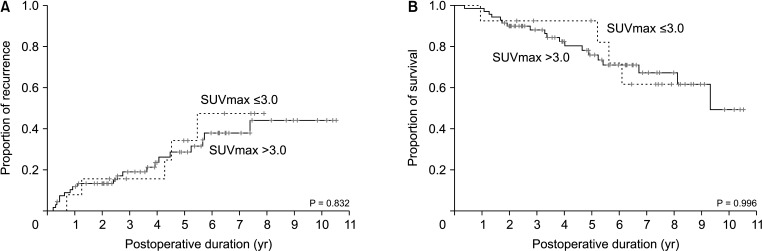
Mean age was 64.4 ± 8.3 years and 76 (75.2%) were male. Anatomical hepatic resection was performed in 99 (98.0%). Concurrent bile duct resection and pancreaticoduodenectomy were performed in 41 (40.6%) and 1 (1.0%), respectively. R0 and R1 resections were performed in 88 (87.1%) and 13 (12.9%), respectively. Low-grade intraepithelial neoplasia and high-grade neoplasia/invasive carcinoma were diagnosed in 19 (18.8%) and 82 (81.2%), respectively. Median FDG-PET maximal standardized uptake values (SUVmax) in low-grade neoplasia and high-grade neoplasia/carcinoma were 3.6 (range, 1.7–7.6) and 5.2 (range, 1.5–18.7) (P = 0.019), respectively. Receiver operating characteristic curve analysis of SUVmax showed area under the curve of 0.674, with sensitivity of 84.2% and specificity of 47.4% at SUVmax cutoff of 3.0. This cutoff had no significant influence on tumor recurrence (P = 0.832) or patient survival (P = 0.996) in patients with IPNB-L of high-grade neoplasia or invasive carcinoma.
Conclusion
IPNB-L is a rare type of biliary neoplasm and encompasses a histological spectrum ranging from benign disease to invasive carcinoma. An FDG-PET SUVmax cutoff of 3.0 appears to effectively discern high-grade neoplasia/ carcinoma from low-grade neoplasia, which will assist with the surgical strategy for these cases.
Figure
Reference
-
1. Nakanuma Y, Sato Y, Harada K, Sasaki M, Xu J, Ikeda H. Pathological classification of intrahepatic cholangiocarcinoma based on a new concept. World J Hepatol. 2010; 2:419–427. PMID: 21191517.
Article2. Jang KT, Hong SM, Lee KT, Lee JG, Choi SH, Heo JS, et al. Intraductal papillary neoplasm of the bile duct associated with Clonorchis sinensis infection. Virchows Arch. 2008; 453:589–598. PMID: 18855009.
Article3. Itatsu K, Zen Y, Ohira S, Ishikawa A, Sato Y, Harada K, et al. Immunohistochemical analysis of the progression of f lat and papillary preneoplastic lesions in intrahepatic cholangiocarcinogenesis in hepatolithiasis. Liver Int. 2007; 27:1174–1184. PMID: 17919228.
Article4. Zen Y, Fujii T, Itatsu K, Nakamura K, Minato H, Kasashima S, et al. Biliary papillary tumors share pathological features with intraductal papillary mucinous neoplasm of the pancreas. Hepatology. 2006; 44:1333–1343. PMID: 17058219.
Article5. Nakanuma Y. A novel approach to biliary tract pathology based on similarities to pancreatic counterparts: is the biliary tract an incomplete pancreas? Pathol Int. 2010; 60:419–429. PMID: 20518896.
Article6. World Health Organization (WHO) Classification of Tumours Editorial Board. WHO classification of tumours, digestive system tumours. 5th ed. International Agency for Research on Cancer: Lyon, France;2019. p. 279–282.7. Liu Y, Zhong X, Yan L, Zheng J, Liu Z, Liang C. Diagnostic performance of CT and MRI in distinguishing intraductal papillary neoplasm of the bile duct from cholangiocarcinoma with intraductal papillary growth. Eur Radiol. 2015; 25:1967–1974. PMID: 25716939.
Article8. Ikeno Y, Seo S, Yamamoto G, Nakamoto Y, Uemoto Y, Fuji H, et al. Usefulness of preoperative 18F-FDG-PET in detecting invasive intraductal papillary neoplasm of the bile duct. Anticancer Res. 2018; 38:3677–3682. PMID: 29848727.
Article9. Hwang S, Lee YJ, Song GW, Park KM, Kim KH, Ahn CS, et al. Prognostic impact of tumor growth type on 7th AJCC staging system for intrahepatic cholangiocarcinoma: a single-center experience of 659 cases. J Gastrointest Surg. 2015; 19:1291–1304. PMID: 25820487.
Article10. Wu SD, Lu CD, Lu CJ, Huang J, Zhou J. Mucin-producing intrahepatic biliary papillomatosis. Surg Today. 2010; 40:845–850. PMID: 20740348.
Article11. Nakanuma Y, Zen Y, Harada K, Ikeda H, Sato Y, Uehara T, et al. Tumorigenesis and phenotypic characteristics of mucin-producing bile duct tumors: an immunohistochemical approach. J Hepatobiliary Pancreat Sci. 2010; 17:211–222. PMID: 19680592.
Article12. Yeh CN, Jan YY, Yeh TS, Hwang TL, Chen MF. Hepatic resection of the intraductal papillary type of peripheral cholangiocarcinoma. Ann Surg Oncol. 2004; 11:606–611. PMID: 15172934.
Article13. Onoe S, Shimoyama Y, Ebata T, Yokoyama Y, Igami T, Sugawara G, et al. Prognostic delineation of papillary cholangiocarcinoma based on the invasive proportion: a single-institution study with 184 patients. Surgery. 2014; 155:280–291. PMID: 24287144.
Article14. Rocha FG, Lee H, Katabi N, DeMatteo RP, Fong Y, D’Angelica MI, et al. Intraductal papillary neoplasm of the bile duct: a biliary equivalent to intraductal papillary mucinous neoplasm of the pancreas? Hepatology. 2012; 56:1352–1360. PMID: 22504729.
Article15. Barton JG, Barrett DA, Maricevich MA, Schnelldorfer T, Wood CM, Smyrk TC, et al. Intraductal papillary mucinous neoplasm of the biliary tract: a real disease? HPB (Oxford). 2009; 11:684–691. PMID: 20495637.
Article16. Park HJ, Kim SY, Kim HJ, Lee SS, Hong GS, Byun JH, et al. Intraductal papillary neoplasm of the bile duct: clinical, imaging, and pathologic features. AJR Am J Roentgenol. 2018; 211:67–75. PMID: 29629808.
Article17. Nakanuma Y, Sato Y, Ojima H, Kanai Y, Aishima S, Yamamoto M, et al. Clinicopathological characterization of so-called “cholangiocarcinoma with intraductal papillary growth” with respect to “intraductal papillary neoplasm of bile duct (IPNB)”. Int J Clin Exp Pathol. 2014; 7:3112–3122. PMID: 25031730.18. Jadvar H, Alavi A, Gambhir SS. 18F-FDG uptake in lung, breast, and colon cancers: molecular biology correlates and disease characterization. J Nucl Med. 2009; 50:1820–1827. PMID: 19837767.
Article19. Seo S, Hatano E, Higashi T, Hara T, Tada M, Tamaki N, et al. Fluorine-18 fluorodeoxyglucose positron emission tomography predicts tumor differentiation, P-glycoprotein expression, and outcome after resection in hepatocellular carcinoma. Clin Cancer Res. 2007; 13(2 Pt 1):427–433. PMID: 17255262.
Article20. Tomimaru Y, Takeda Y, Tatsumi M, Kim T, Kobayashi S, Marubashi S, et al. Utility of 2-[18F] fluoro-2-deoxy-D-glucose positron emission tomography in differential diagnosis of benign and malignant intraductal papillary-mucinous neoplasm of the pancreas. Oncol Rep. 2010; 24:613–620. PMID: 20664965.
Article21. Yamashita YI, Okabe H, Hayashi H, Imai K, Nakagawa S, Nakao Y, et al. Usefulness of 18-FDG PET/CT in detecting malignancy in intraductal papillary mucinous neoplasms of the pancreas. Anticancer Res. 2019; 39:2493–2499. PMID: 31092444.
Article22. Bertagna F, Treglia G, Baiocchi GL, Giubbini R. F18-FDG-PET/CT for evaluation of intraductal papillary mucinous neoplasms (IPMN): a review of the literature. Jpn J Radiol. 2013; 31:229–236. PMID: 23315020.
Article23. Serafini S, Sperti C, Brazzale AR, Cecchin D, Zucchetta P, Pierobon ES, et al. The role of positron emission tomography in clinical management of intraductal papillary mucinous neoplasms of the pancreas. Cancers (Basel). 2020; 12:807.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Intraductal Papillary Mucinous Tumor Simultaneously Involving the Liver and Pancreas: A Case Report
- Intrahepatic and extrahepatic intraductal papillary neoplasms of bile duct
- Extrahepatic Bile Duct Duplication with Intraductal Papillary Neoplasm: A Case Report
- Caroli's disease misdiagnosed as intraductal papillary neoplasm of the bile duct
- Radiological Spectrum of Intraductal Papillary Tumors of the Bile Ducts