Korean J Gastroenterol.
2022 Apr;79(4):170-176. 10.4166/kjg.2022.008.
Expression of ICAM-1 in Blood Vascular Endothelium and Tissues in Human Premalignant Lesion and Gastric/Hepatocellular Carcinomas
- Affiliations
-
- 1Department of Molecular Pathophysiology, Kyungpook National University College of Pharmacy, Daegu, Korea
- 2Department of Pathology, Kyungpook National University Chilgok Hospital, Kyungpook National University School of Medicine, Daegu, Korea
- 3Vessel-Organ Interaction Research Center, VOICE (MRC), Kyungpook National University, Daegu, Korea
- KMID: 2529367
- DOI: http://doi.org/10.4166/kjg.2022.008
Abstract
- Background/Aims
Angiogenesis is essential for the outgrowth and metastasis of tumors. The structure and characteristics of tumor vasculature differ from those of normal vessels. We compared the characteristics of differentially expressed genes in endothelial cells (ECs) isolated from gastric and normal cells.
Methods
Previously, we had isolated pure tumor ECs (TECs) and normal ECs (NECs) from advanced gastric cancer (AGC) lesions and normal mucosal tissues, respectively. Using the oligomer chip platform of the Affymetrix GeneChip technology, genes that were expressed more than three-fold with a significance of p≤0.001 were measured. The intercellular adhesion molecule 1 (ICAM-1) was found to be overexpressed in the TECs compared to the normal gastric ECs. In this study, the upregulation of ICAM-1 was confirmed in cultured TECs by immunofluorescence.
Results
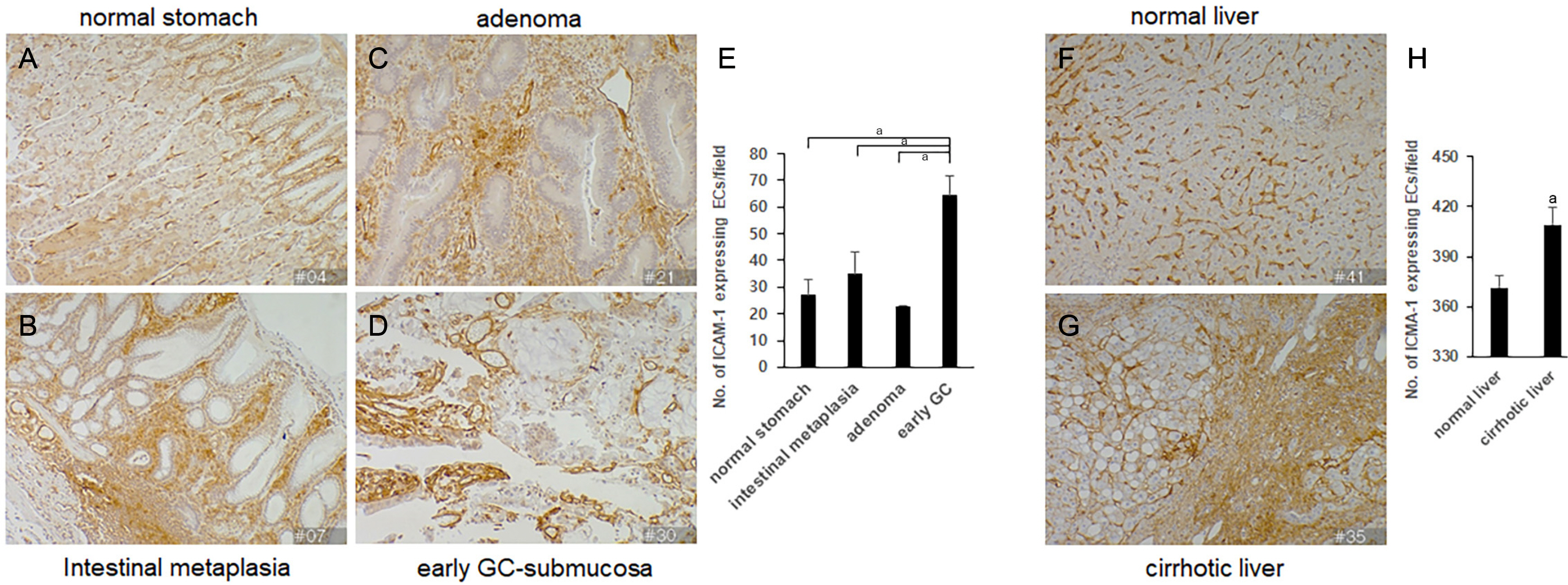
The expression of ICAM-1 was upregulated in the ECs, as well as in the stromal and immune cells, in early human gastric preneoplastic and hepatic fibrotic tissues. Upregulation of ICAM-1 was observed in the TECs, immune cells, and cancer epithelial cells in AGC and hepatocellular carcinoma (HCC). These results suggest that increased ICAM-1 expression in the ECs of the tissue microenvironment progressively contributes to the recruitment of immune cells to promote inflammation, leading to fibrosis and tumorigenesis.
Conclusions
Therefore, upregulated ICAM-1 in the tissues in premalignant gastric diseases or hepatic fibrosis and their malignant cancers could be a promising target for disease prevention and treatment.
Keyword
Figure
Reference
-
1. Hida K, Hida Y, Amin DN, et al. 2004; Tumor-associated endothelial cells with cytogenetic abnormalities. Cancer Res. 64:8249–8255. DOI: 10.1158/0008-5472.CAN-04-1567. PMID: 15548691.
Article2. Kim WH, Lee SH, Jung MH, et al. 2009; Neuropilin2 expressed in gastric cancer endothelial cells increases the proliferation and migration of endothelial cells in response to VEGF. Exp Cell Res. 315:2154–2164. DOI: 10.1016/j.yexcr.2009.04.018. PMID: 19409892.
Article3. Greten FR, Grivennikov SI. 2019; Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. 51:27–41. DOI: 10.1016/j.immuni.2019.06.025. PMID: 31315034. PMCID: PMC6831096.
Article4. Machlowska J, Baj J, Sitarz M, Maciejewski R, Sitarz R. 2020; Gastric cancer: epidemiology, risk factors, classification, genomic characteristics and treatment strategies. Int J Mol Sci. 21:4012. DOI: 10.3390/ijms21114012. PMID: 32512697. PMCID: PMC7312039.
Article5. Rawla P, Barsouk A. 2019; Epidemiology of gastric cancer: global trends, risk factors and prevention. Prz Gastroenterol. 14:26–38. DOI: 10.5114/pg.2018.80001. PMID: 30944675. PMCID: PMC6444111.
Article6. Hsieh HL, Tsai MM. 2019; Tumor progression-dependent angiogenesis in gastric cancer and its potential application. World J Gastrointest Oncol. 11:686–704. DOI: 10.4251/wjgo.v11.i9.686. PMID: 31558974. PMCID: PMC6755109.
Article7. Chen CN, Cheng YM, Lin MT, Hsieh FJ, Lee PH, Chang KJ. 2002; Association of color Doppler vascularity index and microvessel density with survival in patients with gastric cancer. Ann Surg. 235:512–518. DOI: 10.1097/00000658-200204000-00009. PMID: 11923607. PMCID: PMC1422466.
Article8. Gong W, Wang L, Yao JC, et al. 2005; Expression of activated signal transducer and activator of transcription 3 predicts expression of vascular endothelial growth factor in and angiogenic phenotype of human gastric cancer. Clin Cancer Res. 11:1386–1393. DOI: 10.1158/1078-0432.CCR-04-0487. PMID: 15746037.
Article9. Coulon S, Heindryckx F, Geerts A, Van Steenkiste C, Colle I, Van Vlierberghe H. 2011; Angiogenesis in chronic liver disease and its complications. Liver Int. 31:146–162. DOI: 10.1111/j.1478-3231.2010.02369.x. PMID: 21073649.
Article10. Bui TM, Wiesolek HL, Sumagin R. 2020; ICAM-1: a master regulator of cellular responses in inflammation, injury resolution, and tumorigenesis. J Leukoc Biol. 108:787–799. DOI: 10.1002/JLB.2MR0220-549R. PMID: 32182390. PMCID: PMC7977775.
Article11. Ramos TN, Bullard DC, Barnum SR. 2014; ICAM-1: isoforms and phenotypes. J Immunol. 192:4469–4474. DOI: 10.4049/jimmunol.1400135. PMID: 24795464. PMCID: PMC4015451.
Article12. Maruo Y, Gochi A, Kaihara A, et al. 2002; ICAM-1 expression and the soluble ICAM-1 level for evaluating the metastatic potential of gastric cancer. Int J Cancer. 100:486–490. DOI: 10.1002/ijc.10514. PMID: 12115535.
Article13. Fujihara T, Yashiro M, Inoue T, et al. 1999; Decrease in ICAM-1 expression on gastric cancer cells is correlated with lymph node metastasis. Gastric Cancer. 2:221–225. DOI: 10.1007/s101200050067. PMID: 11957102.
Article14. St Croix B, Rago C, Velculescu V, et al. 2000; Genes expressed in human tumor endothelium. Science. 289:1197–1202. DOI: 10.1126/science.289.5482.1197. PMID: 10947988.
Article15. Albini A, Tosetti F, Li VW, Noonan DM, Li WW. 2012; Cancer prevention by targeting angiogenesis. Nat Rev Clin Oncol. 9:498–509. DOI: 10.1038/nrclinonc.2012.120. PMID: 22850752.
Article16. Kim HS, Lim SJ, Park YK. 2009; Anti-angiogenic factor endostatin in osteosarcoma. APMIS. 117:716–723. DOI: 10.1111/j.1600-0463.2009.02524.x. PMID: 19775339.
Article17. Terol MJ, Tormo M, Martinez-Climent JA, et al. 2003; Soluble intercellular adhesion molecule-1 (s-ICAM-1/s-CD54) in diffuse large B-cell lymphoma: association with clinical characteristics and outcome. Ann Oncol. 14:467–474. DOI: 10.1093/annonc/mdg057. PMID: 12598355.
Article18. Hubbard AK, Rothlein R. 2000; Intercellular adhesion molecule-1 (ICAM-1) expression and cell signaling cascades. Free Radic Biol Med. 28:1379–1386. DOI: 10.1016/S0891-5849(00)00223-9.
Article19. Jeong JH, Ojha U, Lee YM. 2021; Pathological angiogenesis and inflammation in tissues. Arch Pharm Res. 44:1–15. DOI: 10.1007/s12272-020-01287-2. PMID: 33230600. PMCID: PMC7682773.
Article20. Azzi S, Hebda JK, Gavard J. 2013; Vascular permeability and drug delivery in cancers. Front Oncol. 3:211. DOI: 10.3389/fonc.2013.00211. PMID: 23967403. PMCID: PMC3744053.
Article21. Jain RK. 2005; Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 307:58–62. DOI: 10.1126/science.1104819. PMID: 15637262.
Article22. Wiesolek HL, Bui TM, Lee JJ, et al. 2020; Intercellular adhesion molecule 1 functions as an efferocytosis receptor in inflammatory macrophages. Am J Pathol. 190:874–885. DOI: 10.1016/j.ajpath.2019.12.006. PMID: 32035057. PMCID: PMC7180595.
Article23. Elliott MR, Koster KM, Murphy PS. 2017; Efferocytosis signaling in the regulation of macrophage inflammatory responses. J Immunol. 198:1387–1394. DOI: 10.4049/jimmunol.1601520. PMID: 28167649. PMCID: PMC5301545.
Article24. Gho YS, Kleinman HK, Sosne G. 1999; Angiogenic activity of human soluble intercellular adhesion molecule-1. Cancer Res. 59:5128–5132.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of Intercellular Adhesion Molecule-l (ICAM-1) in Vascular Endothelium and Keratinocytes of Psoriatic Skin
- Increased Expression of ICAM-1 and VCAM-1 in Human Lupus Nephritis
- Expression of nm23 Protein in Human Gastric Carcinoma: correlation between nm23 expression with the development and metastasis of gastric carcinoma
- Activated platelets induce secretion of interleukin-1beta, monocyte chemotactic protein-1, and macrophage inflammatory protein-1alpha and surface expression of intercellular adhesion molecule-1 on cultured endothelial cells
- Behcet's disease sera containing antiendothelial cell antibodies promote adhesion of T lymphocytes to cultured human dermal microvascular endothelial cells