Endocrinol Metab.
2022 Apr;37(2):290-302. 10.3803/EnM.2021.1343.
Developmental Hypothyroidism Influences the Development of the Entorhinal-Dentate Gyrus Pathway of Rat Offspring
- Affiliations
-
- 1Department of Endocrinology and Metabolism, Institute of Endocrinology, National Health Commission Key Laboratory of Thyroid Diseases, The First Affiliated Hospital of China Medical University, Shenyang, China
- 2Department of Endocrinology, Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, Hangzhou, China
- 3Department of Endocrinology, Chifeng College Affiliated Hospital, Chifeng, China
- KMID: 2529221
- DOI: http://doi.org/10.3803/EnM.2021.1343
Abstract
- Background
Developmental hypothyroidism impairs learning and memory in offspring, which depend on extensive neuronal circuits in the entorhinal cortex, together with the hippocampus and neocortex. The entorhinal-dentate gyrus pathway is the main entrance of memory circuits. We investigated whether developmental hypothyroidism impaired the morphological development of the entorhinal-dentate gyrus pathway.
Methods
We examined the structure and function of the entorhinal-dentate gyrus pathway in response to developmental hypothyroidism induced using 2-mercapto-1-methylimidazole.
Results
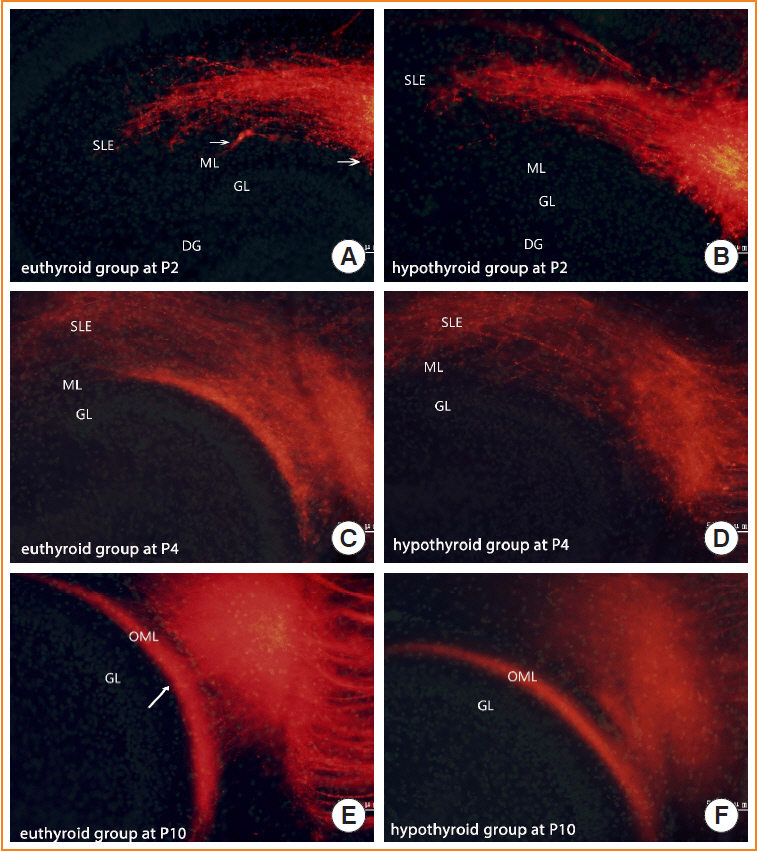
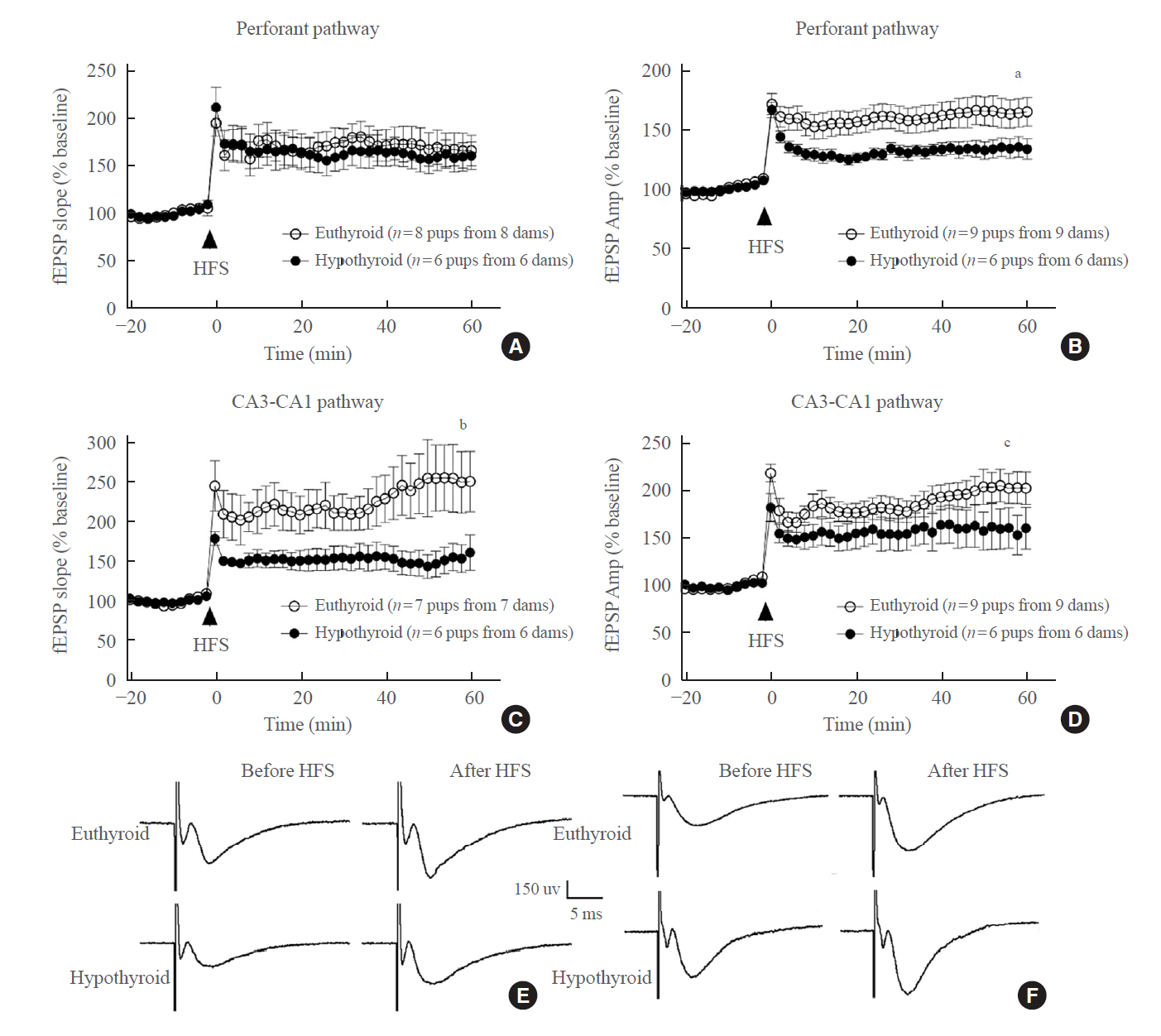
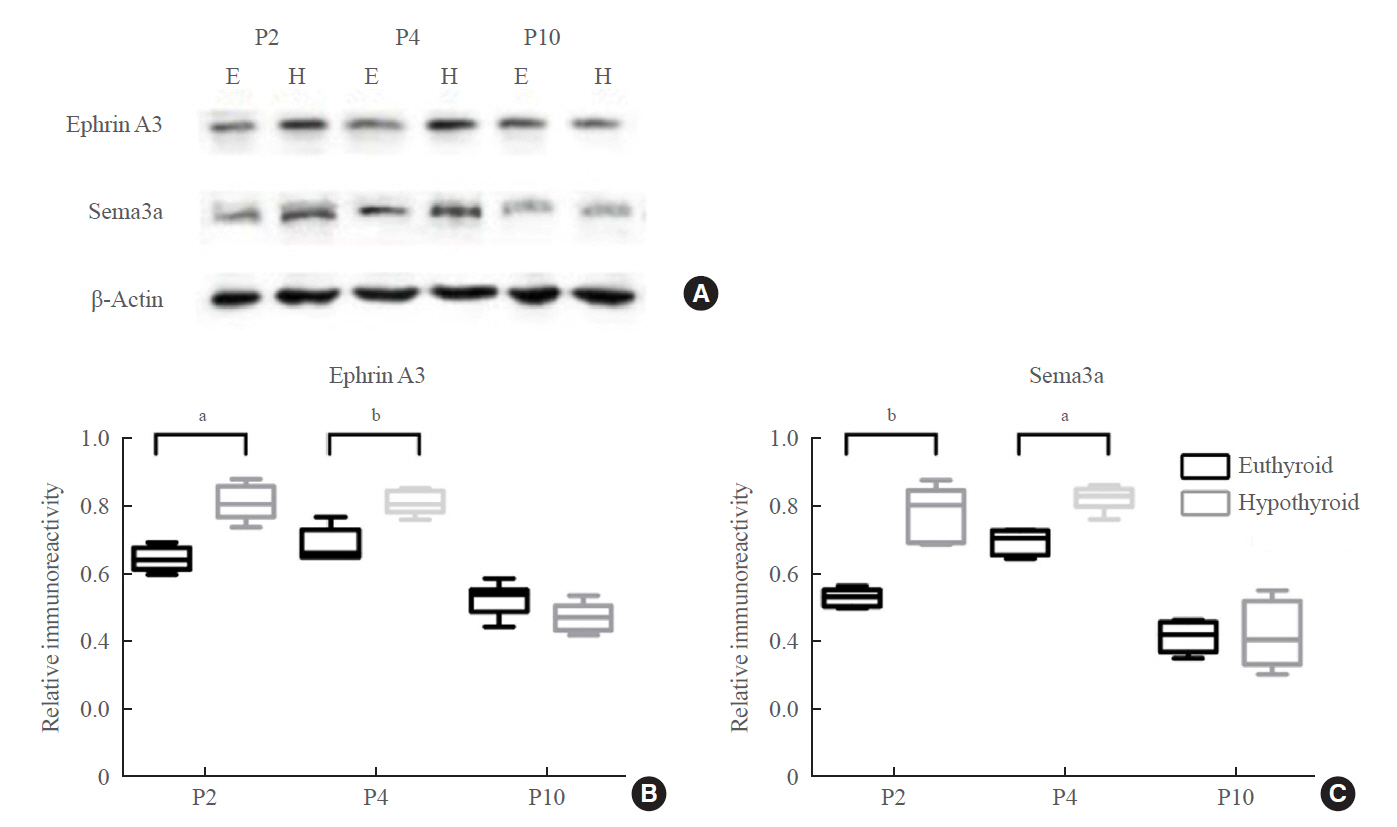
1,1´-Dioctadecyl-3,3,3´,3´-tetramethylindocarbocyanine perchlorate tract tracing indicated that entorhinal axons showed delayed growth in reaching the outer molecular layer of the dentate gyrus at postnatal days 2 and 4 in hypothyroid conditions. The proportion of fibers in the outer molecular layer was significantly smaller in the hypothyroid group than in the euthyroid group at postnatal day 4. At postnatal day 10, the pathway showed a layer-specific distribution in the outer molecular layer, similar to the euthyroid group. However, the projected area of entorhinal axons was smaller in the hypothyroid group than in the euthyroid group. An electrophysiological examination showed that hypothyroidism impaired the long-term potentiation of the perforant and the cornu ammonis 3–cornu ammonis 1 pathways. Many repulsive axon guidance molecules were involved in the formation of the entorhinaldentate gyrus pathway. The hypothyroid group had higher levels of erythropoietin-producing hepatocyte ligand A3 and semaphorin 3A than the euthyroid group.
Conclusion
We demonstrated that developmental hypothyroidism might influence the development of the entorhinal-dentate gyrus pathway, contributing to impaired long-term potentiation. These findings improve our understanding of neural mechanisms for memory function.
Figure
Reference
-
1. Wang W, Teng W, Shan Z, Wang S, Li J, Zhu L, et al. The prevalence of thyroid disorders during early pregnancy in China: the benefits of universal screening in the first trimester of pregnancy. Eur J Endocrinol. 2011; 164:263–8.
Article2. LaFranchi SH, Haddow JE, Hollowell JG. Is thyroid inadequacy during gestation a risk factor for adverse pregnancy and developmental outcomes? Thyroid. 2005; 15:60–71.
Article3. Ahmed OM, El-Gareib AW, El-Bakry AM, Abd El-Tawab SM, Ahmed RG. Thyroid hormones states and brain development interactions. Int J Dev Neurosci. 2008; 26:147–209.
Article4. Bernal J, Guadano-Ferraz A, Morte B. Perspectives in the study of thyroid hormone action on brain development and function. Thyroid. 2003; 13:1005–12.
Article5. Giese KP, Fedorov NB, Filipkowski RK, Silva AJ. Autophosphorylation at Thr286 of the alpha calcium-calmodulin kinase II in LTP and learning. Science. 1998; 279:870–3.
Article6. Rogan MT, Staubli UV, LeDoux JE. Fear conditioning induces associative long-term potentiation in the amygdala. Nature. 1997; 390:604–7.
Article7. Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006; 313:1093–7.
Article8. Miller S, Mayford M. Cellular and molecular mechanisms of memory: the LTP connection. Curr Opin Genet Dev. 1999; 9:333–7.
Article9. Lynch MA. Long-term potentiation and memory. Physiol Rev. 2004; 84:87–136.
Article10. Nicoll RA. A brief history of long-term potentiation. Neuron. 2017; 93:281–90.
Article11. Gilbert ME, Sui L. Dose-dependent reductions in spatial learning and synaptic function in the dentate gyrus of adult rats following developmental thyroid hormone insufficiency. Brain Res. 2006; 1069:10–22.
Article12. Opazo MC, Gianini A, Pancetti F, Azkcona G, Alarcon L, Lizana R, et al. Maternal hypothyroxinemia impairs spatial learning and synaptic nature and function in the offspring. Endocrinology. 2008; 149:5097–106.
Article13. Alzoubi KH, Gerges NZ, Aleisa AM, Alkadhi KA. Levothyroxin restores hypothyroidism-induced impairment of hippocampus-dependent learning and memory: behavioral, electrophysiological, and molecular studies. Hippocampus. 2009; 19:66–78.
Article14. Zhang Y, Fan Y, Yu X, Wang X, Bao S, Li J, et al. Maternal subclinical hypothyroidism impairs neurodevelopment in rat offspring by inhibiting the CREB signaling pathway. Mol Neurobiol. 2015; 52:432–41.
Article15. Gilbert ME, Paczkowski C. Propylthiouracil (PTU)-induced hypothyroidism in the developing rat impairs synaptic transmission and plasticity in the dentate gyrus of the adult hippocampus. Brain Res Dev Brain Res. 2003; 145:19–29.
Article16. McClelland JL, McNaughton BL, O’Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol Rev. 1995; 102:419–57.
Article17. Steffenach HA, Witter M, Moser MB, Moser EI. Spatial memory in the rat requires the dorsolateral band of the entorhinal cortex. Neuron. 2005; 45:301–13.
Article18. Basu J, Siegelbaum SA. The corticohippocampal circuit, synaptic plasticity, and memory. Cold Spring Harb Perspect Biol. 2015; 7:a021733.
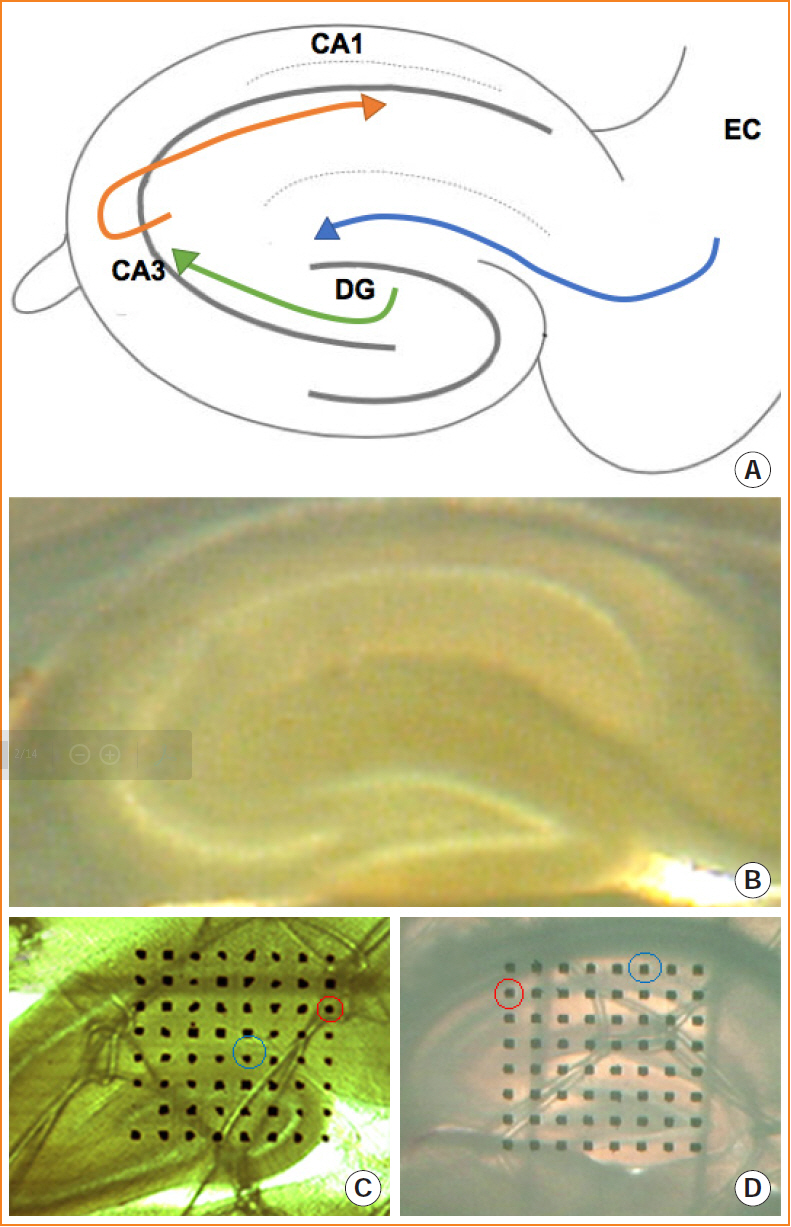
Article19. Witter MP. The perforant path: projections from the entorhinal cortex to the dentate gyrus. Prog Brain Res. 2007; 163:43–61.
Article20. Ahmed OJ, Mehta MR. The hippocampal rate code: anatomy, physiology and theory. Trends Neurosci. 2009; 32:329–38.
Article21. Bartesaghi R, Gessi T, Migliore M. Input-output relations in the entorhinal-hippocampal-entorhinal loop: entorhinal cortex and dentate gyrus. Hippocampus. 1995; 5:440–51.
Article22. Kesner RP, Gilbert PE, Wallenstein GV. Testing neural network models of memory with behavioral experiments. Curr Opin Neurobiol. 2000; 10:260–5.
Article23. Bernal J. Thyroid hormones and brain development. Vitam Horm. 2005; 71:95–122.
Article24. Koromilas C, Liapi C, Schulpis KH, Kalafatakis K, Zarros A, Tsakiris S. Structural and functional alterations in the hippocampus due to hypothyroidism. Metab Brain Dis. 2010; 25:339–54.
Article25. Auso E, Lavado-Autric R, Cuevas E, Del Rey FE, Morreale De Escobar G, et al. A moderate and transient deficiency of maternal thyroid function at the beginning of fetal neocorticogenesis alters neuronal migration. Endocrinology. 2004; 145:4037–47.
Article26. Wong CC, Leung MS. Effects of neonatal hypothyroidism on the expressions of growth cone proteins and axon guidance molecules related genes in the hippocampus. Mol Cell Endocrinol. 2001; 184:143–50.
Article27. Alvarez-Dolado M, Figueroa A, Kozlov S, Sonderegger P, Furley AJ, Munoz A. Thyroid hormone regulates TAG-1 expression in the developing rat brain. Eur J Neurosci. 2001; 14:1209–18.
Article28. Tamamaki N. Organization of the entorhinal projection to the rat dentate gyrus revealed by Dil anterograde labeling. Exp Brain Res. 1997; 116:250–8.
Article29. He Y, Liu MG, Gong KR, Chen J. Differential effects of long and short train theta burst stimulation on LTP induction in rat anterior cingulate cortex slices: multi-electrode array recordings. Neurosci Bull. 2009; 25:309–18.
Article30. Miao HH, Li XH, Chen QY, Zhuo M. Calcium-stimulated adenylyl cyclase subtype 1 is required for presynaptic longterm potentiation in the insular cortex of adult mice. Mol Pain. 2019; 15:1744806919842961.
Article31. Deng JB, Yu DM, Wu P, Li MS. The tracing study of developing entorhino-hippocampal pathway. Int J Dev Neurosci. 2007; 25:251–8.
Article32. Berbel P, Guadano-Ferraz A, Martinez M, Quiles JA, Balboa R, Innocenti GM. Organization of auditory callosal connections in hypothyroid adult rats. Eur J Neurosci. 1993; 5:1465–78.
Article33. Kitamura T. Driving and regulating temporal association learning coordinated by entorhinal-hippocampal network. Neurosci Res. 2017; 121:1–6.
Article34. Godement P, Vanselow J, Thanos S, Bonhoeffer F. A study in developing visual systems with a new method of staining neurones and their processes in fixed tissue. Development. 1987; 101:697–713.
Article35. Heilingoetter CL, Jensen MB. Histological methods for ex vivo axon tracing: a systematic review. Neurol Res. 2016; 38:561–9.36. Chen BK, Miller SM, Mantilla CB, Gross L, Yaszemski MJ, Windebank AJ. Optimizing conditions and avoiding pitfalls for prolonged axonal tracing with carbocyanine dyes in fixed rat spinal cords. J Neurosci Methods. 2006; 154:256–63.
Article37. Auso E, Cases O, Fouquet C, Camacho M, Garcia-Velasco JV, Gaspar P, et al. Protracted expression of serotonin transporter and altered thalamocortical projections in the barrelfield of hypothyroid rats. Eur J Neurosci. 2001; 14:1968–80.
Article38. Silva AJ. Molecular and cellular cognitive studies of the role of synaptic plasticity in memory. J Neurobiol. 2003; 54:224–37.
Article39. Madeira MD, Paula-Barbosa MM. Reorganization of mossy fiber synapses in male and female hypothyroid rats: a stereological study. J Comp Neurol. 1993; 337:334–52.
Article40. Dong J, Yin H, Liu W, Wang P, Jiang Y, Chen J. Congenital iodine deficiency and hypothyroidism impair LTP and decrease C-fos and C-jun expression in rat hippocampus. Neurotoxicology. 2005; 26:417–26.
Article41. Duffy CJ, Teyler TJ. Development of potentiation in the dentate gyrus of rat: physiology and anatomy. Brain Res Bull. 1978; 3:425–30.
Article42. Hussain RJ, Carpenter DO. Development of synaptic responses and plasticity at the SC-CA1 and MF-CA3 synapses in rat hippocampus. Cell Mol Neurobiol. 2001; 21:357–68.43. Yasuda H, Barth AL, Stellwagen D, Malenka RC. A developmental switch in the signaling cascades for LTP induction. Nat Neurosci. 2003; 6:15–6.
Article44. Skutella T, Nitsch R. New molecules for hippocampal development. Trends Neurosci. 2001; 24:107–13.
Article45. Brinks H, Conrad S, Vogt J, Oldekamp J, Sierra A, Deitinghoff L, et al. The repulsive guidance molecule RGMa is involved in the formation of afferent connections in the dentate gyrus. J Neurosci. 2004; 24:3862–9.
Article46. Chedotal A, Del Rio JA, Ruiz M, He Z, Borrell V, de Castro F, et al. Semaphorins III and IV repel hippocampal axons via two distinct receptors. Development. 1998; 125:4313–23.
Article47. Stein E, Savaskan NE, Ninnemann O, Nitsch R, Zhou R, Skutella T. A role for the Eph ligand ephrin-A3 in entorhino-hippocampal axon targeting. J Neurosci. 1999; 19:8885–93.
Article48. Santisteban P, Bernal J. Thyroid development and effect on the nervous system. Rev Endocr Metab Disord. 2005; 6:217–28.
Article49. Bernal J. Action of thyroid hormone in brain. J Endocrinol Invest. 2002; 25:268–88.
Article50. Ceranik K, Zhao S, Frotscher M. Development of the entorhino-hippocampal projection: guidance by Cajal-Retzius cell axons. Ann N Y Acad Sci. 2000; 911:43–54.
Article51. Zhao S, Chai X, Forster E, Frotscher M. Reelin is a positional signal for the lamination of dentate granule cells. Development. 2004; 131:5117–25.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunocytochemical Study on the Changes of Cell-Death Controlling Factors in the Hippocampal Formation and Entorhinal Cortex of Aged Rats
- Calcium Influx is Responsible for Afterdepolarizations in Rat Hippocampal Dentate Granule Cells
- Expression Changes of c-Fos Protein of Rat Brain Following Pentylenetetrazol-induced Seizures
- Differential expression levels of synaptophysin through developmental stages in hippocampal region of mouse brain
- Developmental mRNA Expression of cdk5 and its Putative Regulators, p67 and p35, in Rat Brain