Cancer Res Treat.
2022 Apr;54(2):554-562. 10.4143/crt.2021.375.
Effectiveness of Concomitant Chemoradiotherapy with Gemcitabine in Locally Advanced Cervical Cancer Patients with Comorbidities
- Affiliations
-
- 1National Network of Cancer Records, Instituto Nacional de Cancerología, Mexico City, Mexico
- 2Department of Clinical Research, Instituto Nacional de Cancerología, Mexico City, Mexico
- 3Consejo Nacional de Ciencia y Tecnología (CONACyT)- Department of Clinical Research, Instituto Nacional de Cancerología, Mexico City, Mexico
- 4Hospital Regional No. 2 El Marques, Instituto Mexicano del Seguro Social, Queretaro, Mexico
- 5Research Unit in Metabolic Diseases, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico City, Mexico
- KMID: 2528224
- DOI: http://doi.org/10.4143/crt.2021.375
Abstract
- Purpose
The standard treatment for locally advanced cervical cancer (LACC) is concomitant chemoradiotherapy with cisplatin (CDDP) followed by brachytherapy. The presence of comorbidities are risk factors for nephrotoxicity and are associated with lower survival. Gemcitabine is a radiosensitizing drug that has shown efficacy and safety in this context. The effectiveness of concomitant chemoradiotherapy with gemcitabine was evaluated versus cisplatin in LACC patients with comorbidities and preserved renal function.
Materials and Methods
An observational, longitudinal and paired study was carried out that included patients treated between February 2003 and December 2015. The primary objectives were to evaluate response rates, progression-free survival, and overall survival; the secondary objectives were to evaluate toxicity and renal function.
Results
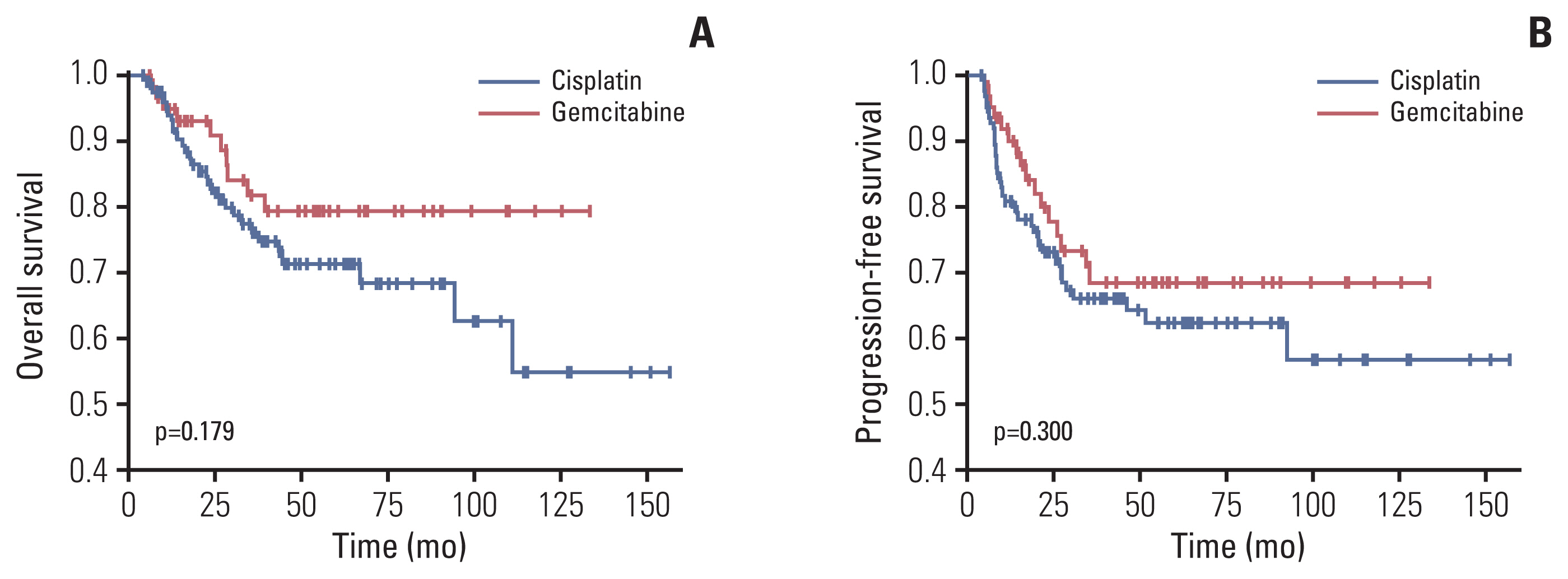
Sixty-three patients treated with gemcitabine at 300 mg/m2 weekly and 126 patients treated with CDDP 40 mg/m2 weekly were included. There were no significant differences in response rates and survival rates. Treatment with cisplatin presented a higher frequency of hematological toxicities, while gemcitabine presented a higher frequency of gastrointestinal toxicities. A decrease in glomerular filtration rate (GFR; baseline vs. 1-year post-treatment) was observed in the cisplatin group (p=0.002), while not in the gemcitabine group (p=0.667). In a multivariate analysis, it is observed that only CDDP correlates with the decrease in GFR (hazard ratio, 2.42; p=0.012).
Conclusion
In LACC patients with comorbidities, gemcitabine and CDDP show the same efficacy, with different toxicity profiles. Treatment with cisplatin is associated with a significant decrease in GFR during follow-up, compared to treatment with gemcitabine that does not decrease it.
Figure
Reference
-
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68:394–424.
Article2. Rizo Ríos P, Sierra Colindres MI, Vázquez Pinon G, Cano Guadiana MC, Meneses Garcia A, Mohar A. Cancer hospital registry: cancer compendium 2000–2004. Cancerologia. 2007; 2:208–87.3. Rose PG, Bundy BN, Watkins EB, Thigpen JT, Deppe G, Maiman MA, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999; 340:1144–53.
Article4. Datta NR, Stutz E, Liu M, Rogers S, Klingbiel D, Siebenhuner A, et al. Concurrent chemoradiotherapy vs. radiotherapy alone in locally advanced cervix cancer: a systematic review and meta-analysis. Gynecol Oncol. 2017; 145:374–85.5. Tan LT, Zahra M. Long-term survival and late toxicity after chemoradiotherapy for cervical cancer: the Addenbrooke’s experience. Clin Oncol (R Coll Radiol). 2008; 20:358–64.6. Niho S, Yamanaka T, Umemura S, Matsumoto S, Yoh K, Goto K, et al. Renal toxicity caused by brand-name versus generic cisplatin: a comparative analysis. Jpn J Clin Oncol. 2013; 43:390–5.
Article7. Mizuno T, Ishikawa K, Sato W, Koike T, Kushida M, Miyagawa Y, et al. The risk factors of severe acute kidney injury induced by cisplatin. Oncology. 2013; 85:364–9.
Article8. Mathe C, Bohacs A, Duffek L, Lukacsovits J, Komlosi ZI, Szondy K, et al. Cisplatin nephrotoxicity aggravated by cardiovascular disease and diabetes in lung cancer patients. Eur Respir J. 2011; 37:888–94.
Article9. Zhao L, Leung LH, Wang J, Li H, Che J, Liu L, et al. Association between Charlson comorbidity index score and outcome in patients with stage IIIB–IV non-small cell lung cancer. BMC Pulm Med. 2017; 17:112.
Article10. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987; 40:373–83.
Article11. Romero-Martinez M, Shamah-Levy T, Vielma-Orozco E, Heredia-Hernandez O, Mojica-Cuevas J, Cuevas-Nasu L, et al. National Health and Nutrition Survey 2018–19: methodology and perspectives. Salud Publica Mex. 2019; 61:917–23.12. Nam EJ, Lee M, Yim GW, Kim JH, Kim S, Kim SW, et al. Comparison of carboplatin- and cisplatin-based concurrent chemoradiotherapy in locally advanced cervical cancer patients with morbidity risks. Oncologist. 2013; 18:843–9.
Article13. Coronel JA, Cetina Ldel C, Cantu D, Cerezo O, Hernandez CS, Rivera L, et al. A randomized comparison of cisplatin and oral vinorelbine as radiosensitizers in aged or comorbid locally advanced cervical cancer patients. Int J Gynecol Cancer. 2013; 23:884–9.
Article14. Csoka K, Liliemark J, Larsson R, Nygren P. Evaluation of the cytotoxic activity of gemcitabine in primary cultures of tumor cells from patients with hematologic or solid tumors. Semin Oncol. 1995; 22:47–53.15. McCormack M, Thomas H. A phase Ib study of gemcitabine (GEM) and concurrent radiotherapy (RT) in carcinoma of the cervix. Ann Oncol. 2000; 11(Suppl 4):88–9.16. Boualga K, Aksil N, Ayad M, Hasnaoui N, Moussaoui D. Phase I/II study of gemcitabine (GEM) and concomitant radiotherapy (RT) in locally advanced carcinoma of the cervix (LACC). J Clin Oncol. 2005; 23(16 Suppl):5142.
Article17. Pattaranutaporn P, Thirapakawong C, Chansilpa Y, Therasakvichya S, Ieumwananontachai N, Thephamongkhol K. Phase II study of concurrent gemcitabine and radiotherapy in locally advanced stage IIIB cervical carcinoma. Gynecol Oncol. 2001; 81:404–7.
Article18. Verma AK, Arya AK, Kumar M, Kumar A, Gupta S, Sharma D, et al. Weekly cisplatin or gemcitabine concomitant with radiation in the management of locally advanced carcinoma cervix: results from an observational study. J Gynecol Oncol. 2009; 20:221–6.
Article19. Kundu S, Basu S, Acharya S, Dastidar A, Roy A. Chemoradiation in locally advanced cervical cancer: a randomized trial. Indian J Med Paediatr Oncol. 2008; 29:12–8.
Article20. Cetina L, Rivera L, Candelaria M, de la Garza J, Duenas-Gonzalez A. Chemoradiation with gemcitabine for cervical cancer in patients with renal failure. Anticancer Drugs. 2004; 15:761–6.
Article21. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009; 150:604–12.
Article22. Piccirillo JF, Tierney RM, Costas I, Grove L, Spitznagel EL Jr. Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA. 2004; 291:2441–7.
Article23. Lee L, Cheung WY, Atkinson E, Krzyzanowska MK. Impact of comorbidity on chemotherapy use and outcomes in solid tumors: a systematic review. J Clin Oncol. 2011; 29:106–17.
Article24. Yancik R. Cancer burden in the aged: an epidemiologic and demographic overview. Cancer. 1997; 80:1273–83.25. Sarfati D, Koczwara B, Jackson C. The impact of comorbidity on cancer and its treatment. CA Cancer J Clin. 2016; 66:337–50.
Article26. Ferrandina G, Lucidi A, Paglia A, Corrado G, Macchia G, Tagliaferri L, et al. Role of comorbidities in locally advanced cervical cancer patients administered preoperative chemoradiation: impact on outcome and treatment-related complications. Eur J Surg Oncol. 2012; 38:238–44.
Article27. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45:228–47.
Article28. Venook AP, Egorin MJ, Rosner GL, Hollis D, Mani S, Hawkins M, et al. Phase I and pharmacokinetic trial of gemcitabine in patients with hepatic or renal dysfunction: Cancer and Leukemia Group B 9565. J Clin Oncol. 2000; 18:2780–7.
Article29. Dawson LA, Kavanagh BD, Paulino AC, Das SK, Miften M, Li XA, et al. Radiation-associated kidney injury. Int J Radiat Oncol Biol Phys. 2010; 76:S108–15.
Article30. Pabla N, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int. 2008; 73:994–1007.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Long Term Complete Response of Unresectable Locally Advanced Pancreatic Cancer after CCRT and Gemcitabine Chemotherapy
- Adjuvant hysterectomy in patients with locally advanced cervical cancer treated with concurrent chemoradiotherapy
- The ideal strategies of chemotherapy for the treatment of cervical cancer
- The Effects and Surgical Morbidity of Preoperative Combined Chemoradiotherapy for Locally Advanced Rectal Cancer
- Surgical issues in locally advanced rectal cancer treated by preoperative chemoradiotherapy