Korean J Ophthalmol.
2007 Mar;21(1):21-27. 10.3341/kjo.2007.21.1.21.
Direct Detection of Reactive Nitrogen Species in Experimental Autoimmune Uveitis
- Affiliations
-
- 1Department of Ophthalmology & Laboratory of Visual Science, Daejon St. Marys Hospital, Daejon, Korea. sunrbae@yahoo.com
- 2Department of Ophthalmology, Keck School of Medicine, the University of Southern California, Los Angeles, CA.
- 3Department of Molecular Pharmacology and Toxicology, Keck School of Medicine, the University of Southern California, Los Angeles, CA.
- 4Department of Chemistry and Chemical Engineering, California Institute of Technology, Pasadena, CA.
- KMID: 1105026
- DOI: http://doi.org/10.3341/kjo.2007.21.1.21
Abstract
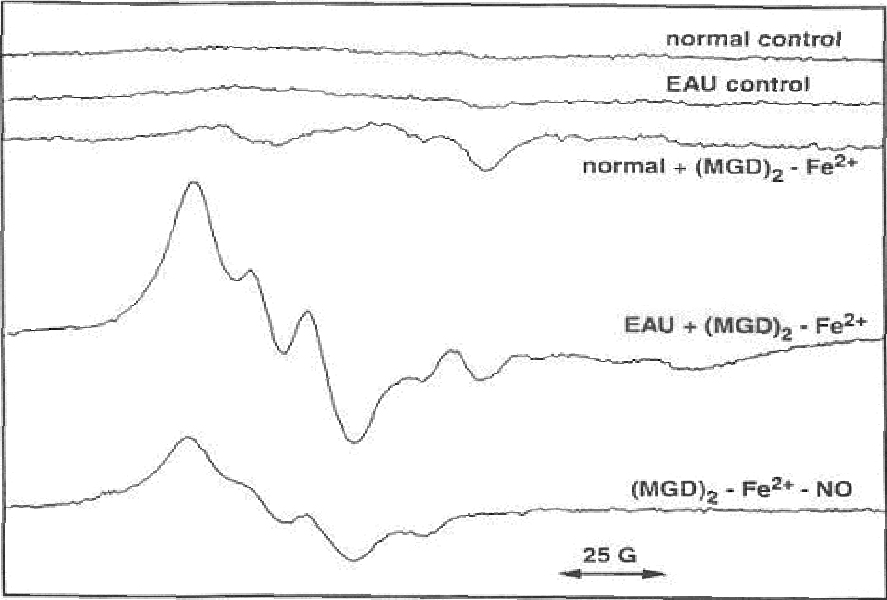
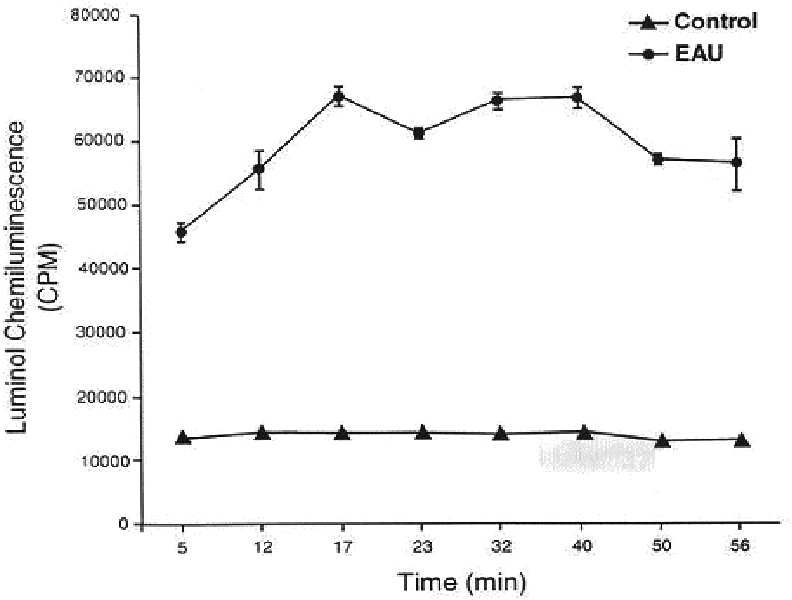
- PURPOSE: Demonstrate unequivocally the generation of nitric oxide in experimental autoimmune uveoretinitis by electron spin resonance spectroscopy (ESR) using ferrous iron complex of N-methyl-D-glucamine dithiocarbamate, (MGD)2-Fe2+, as a spin trap. METHODS: Experimental autoimmune uveitis was induced in Lewis rats, and at the peak of the intraocular inflammation, the animals received intravitreous injections of the spin trap. The retina and choroid dissected from the enucleated globes were subjected to ESR. Similarly, the retina and choroid obtained at the peak of experimental autoimmune uveo-retinitis (EAU) were placed in a vial containing luminal, and chemiluminescence was counted on a Packard liquid scintillation analyzer. RESULTS: The ESR three-line spectrum (g=2.04; a(N)=12.5 G) obtained was characteristic of the adduct [(MGD)2-Fe2+-NO]. The majority of this signal was eliminated by the inducible nitric oxide synthase (iNOS) specific inhibitor aminoguanidine injected inflamed retina was detected when compared with that of the non inflamed controls. The chemiluminescent activity was further increased two-fold by the addition of bicarbonate to the inflamed retina; the phenomenon is attributable only to the presence of a high steady-state concentration of peroxynitrite. CONCLUSIONS: The study shows an unequivocal presence of nitric oxide in EAU retina and choroid and the generation of peroxynitrite. High levels of these reactive nitrogen species generated in the inflamed retina and choroids are certain to cause irreversible tissue damage, especially at the susceptible sites such as photoreceptors.
Keyword
MeSH Terms
-
Uveitis/immunology/*metabolism
Thiocarbamates
Spin Trapping
Spin Labels
Sorbitol/analogs & derivatives
Retina/metabolism
Reactive Nitrogen Species/*metabolism
Rats, Inbred Lew
Rats
Peptide Fragments/immunology
Humans
Electron Spin Resonance Spectroscopy
Choroid/metabolism
Autoimmune Diseases/immunology/*metabolism
Arrestin/immunology
Animals
Figure
Reference
-
1. Rao NA. Role of oxygen free radicals in retinal damage associated with experimental uveitis. Trans Am Ophthalmol Soc. 1990. 88:797–850.2. Rao NA, Wu GS. Free radical mediated photoreceptor damage in uveitis. Progress in Retina and Eye Res. 2000. 19:41–68.3. Zhang J, Wu GS, Rao NA. Role of nitric oxide (NO) in experimental autoimmune uveitis (EAU). Invest Ophthalmol Vis Sci. 1993. 1000:abstr.4. Nathan C, Xie Q-W. Nitric oxide synthases: roles, tolls, and controls. Cell. 1994. 78:915–918.5. Zhang J, Wu LY, Wu GS, et al. Differential expression of nitric oxide synthase in experimental uveoretinitis. Invest Ophthalmol Vis Sci. 1999. 40:1899–1905.6. Jacquemin E, de Kozak Y, Thillaye B, et al. Expression of inducible nitric oxide synthase in the eye from endotoxin-induced uveitis rats. Invest Ophthalmol Vis Sci. 1996. 37:1187–1196.7. Schmidt HHHW, Hofmann H, Schindler U, et al. No ˙NO from NO synthase. Proc Natl Acad Sci USA. 1996. 93:14492–14497.8. Komarov AM, Wink DA, Feelisch M, et al. Electron paramagnetic resonance spectroscopy using N-metyl-D-glucamine dithiocarbamate iron cannot discriminate between nitric oxide synthase. Free Radic Biol Med. 2000. 28:739–742.9. Lai CS, Komarov AM. Spin trapping of nitric oxide produced in vivo in septic-shock mice. FEBSL Lett. 1994. 345:120–124.10. Zweier JL, Wang P, Samouilov A, et al. Enzyme-independent formation of nitric oxide in biological tissues. Nature Med. 1995. 1:804–809.11. Komarov AM, Kramer JH, Mak IT, et al. EPR detection of endogenous nitric oxide in postischemic heart using lipid and aqueous-soluble dithiocarbamate-iron complexes. Mol Cell Biochem. 1997. 175:91–97.12. Obolenskaya M, Vanin AF, et al. EPR evidence of nitric oxide production by the regenerating rat liver. Biochem Biophys Res Commun. 1994. 571–576.13. Komarov AM, Lai CS. Detection of nitric oxide production in mice by spin-trapping electron paramagnetic resonance spectroscopy. Biochim Biophys Acta. 1995. 1272:29–36.14. Bune AJ, Shergill JK, Cammack R, et al. L-arginine depletion by arginase reduces nitric oxide production in endotoxemic shock: an electron paramagnetic resonance study. FEBS Lett. 1995. 366:127–130.15. Allen RC. Phagocytic leukocyte oxygeneration activities and chemiluminescence: a kinetic approach to analysis. Methods Enzymol. 1986. 133:449–493.16. Merenyi G, Lind J, Eriksen TE. The reactivity of superoxide (O2-)and its ability to induce chemiluminescence with luminol. Photochem Photobiol. 1985. 41:203–208.17. Radi R, Rubbo H, Thomson L, et al. Luminol chemiluminescence using xanthine and hypoxanthine as xanthine oxidase substrates. J Free Rad Biol Med. 1990. 8:121–126.18. Archer SL, Nelson DP, Weir EK. Simultaneous measurement of O2 radicals and pulmonary vascular reactivity in rat lung. J Appl Physiol. 1989. 67:1903–1911.19. Dowling EJ, Symons AM, Parke DV. Free radical production at the site of an acute inflammatory reaction as measured by chemiluminescence. Agents Actions. 1986. 19:203–207.20. Beckman JS, Beckman TW, Chen J, et al. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci USA. 1990. 87:1620–1624.21. Radi R, Cosgrove TP, Beckman JS, et al. Peroxynitrite-induced luminol chemiluminescence. Biochem J. 1993. 290:51–57.22. Wang P, Zweier JL. Measurement of nitric oxide and peroxynitrite generation in the postischemic heart. Evidence for peroxynitrite-mediated reperfusion injury. J Biol Chem. 1996. 271:29223–29230.23. Zweier JL, Wang P, Kuppusamy P. Direct measurement of nitric oxide generation in the ischemic heart using electron paramagnetic resonance spectroscopy. J Biol Chem. 1995. 270:304–307.24. Xia Y, Zweier JL. Direct measurement of nitric oxide generation from nitric oxide synthase. Proc Natl Acad Sci USA. 1997. 94:12705–12710.25. Mulsch A, Vanin A, Mordvintcev P, et al. NO accounts completely for the oxyenated nitrogen species generated by enzymic L-arginine oxygenation. Biochem J. 1992. 288:597–603.26. Kubrina LN, Mikoyan VD, Mordvintcev PI, et al. Iron potentiates bacterial lipopolysaccharide-induced nitric oxide formation in animal organs. Biochim Biophys Acta. 1993. 1176:240–244.27. Corbett JA, McDaniel ML. Selective inhibition of inducible nitric oxide synthase by aminoguanidine. Methods Enzymol. 1996. 268:398–408.28. Shinobu LA, Jones SG, Jones MM. Sodium N-methyl-D-glucamine dithiocarbamate and cadmium intoxication. Acta Pharmacol Toxicol (Copenh). 1984. 54:189–194.29. Denicola A, Freeman BA, Trujillo M, et al. Peroxynitrite reaction with carbon dioxide/bicarbonate: kinetics and influence on peroxynitrite-mediated oxidations. Arch Biochem Biophys. 1996. 333:49–58.30. Gow A, Duran D, Thom SR, et al. Carbon dioxide enhancement of peroxynitrite-mediated tyrosine nitration. Arch Biochem Biophys. 1996. 333:42–48.31. Zhang H, Joseph J, Felix C, et al. Bicarbonate enhances the hydroxylation, nitration, and peroxidation reactions catalyzed by copper, zinc superoxide dismutase. Intermediacy of carbonate anion radical. J Biol Chem. 2000. 275:14038–14045.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Reactive Oxygen and Nitrogen Species in Pathogenesis of Vascular Complications of Diabetes
- Effects of Cyclosporin A on the Recurrence in Experimental Autoimmune Anterior Uveitis
- Rhamnazin inhibits LPS-induced inflammation and ROS/RNS in raw macrophages
- Clinical Applications of Antioxidants
- The Antioxidative and Antimicrobial Effects of Gloiopeltis Tenax