J Pathol Transl Med.
2022 Mar;56(2):97-102. 10.4132/jptm.2021.10.28.
Colorectal adenocarcinoma with enteroblastic differentiation: diagnostic challenges of a rare case encountered in clinical practice
- Affiliations
-
- 1Department of Pathology, Wayne State University School of Medicine/Detroit Medical Center, Detroit, MI, USA
- 2Larkin Community Hospital, South Miami, FL, USA
- 3Ascension St. John Hospital, Detroit, MI, USA
- 4Michigan State University, East Lansing, MI, USA
- KMID: 2527194
- DOI: http://doi.org/10.4132/jptm.2021.10.28
Abstract
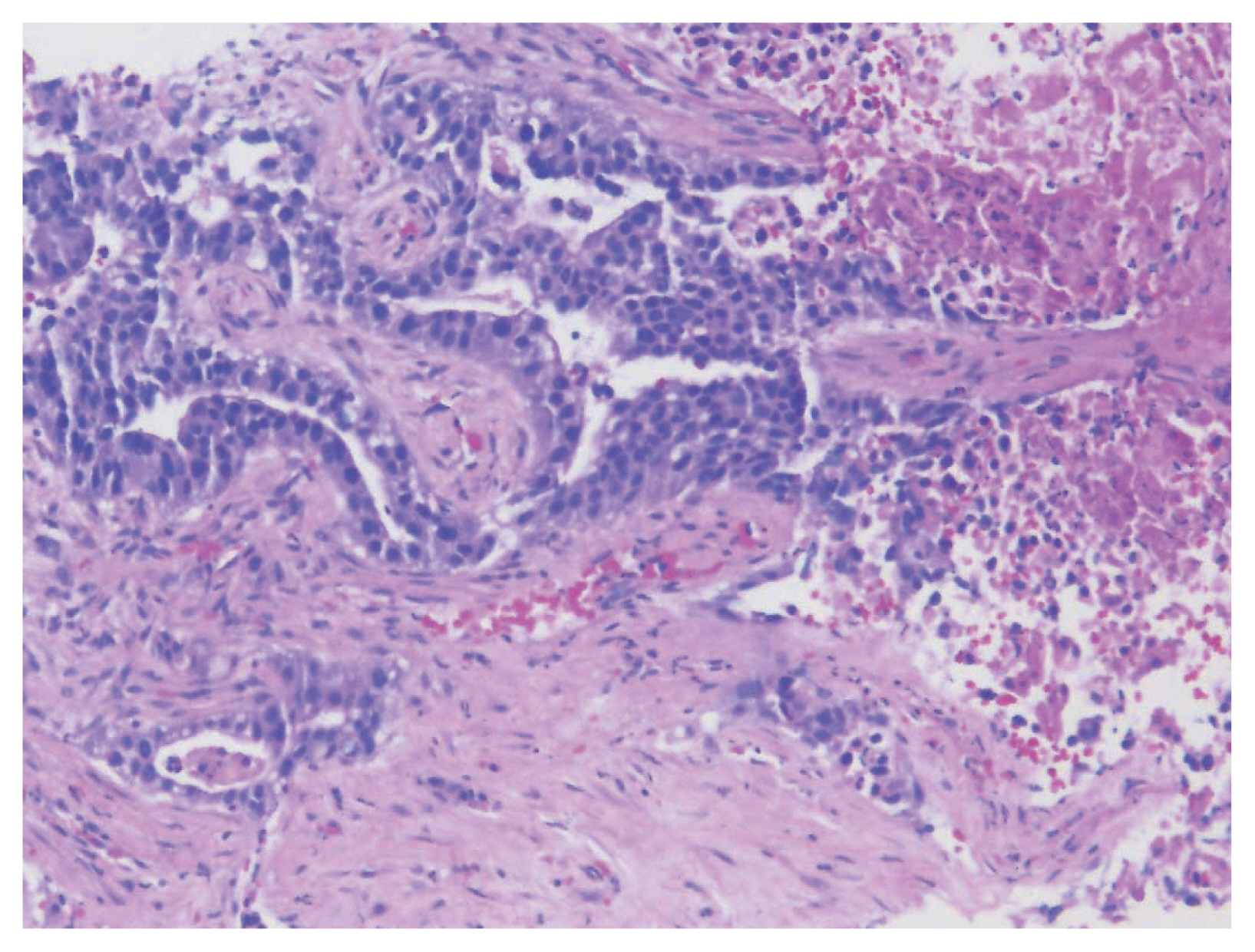
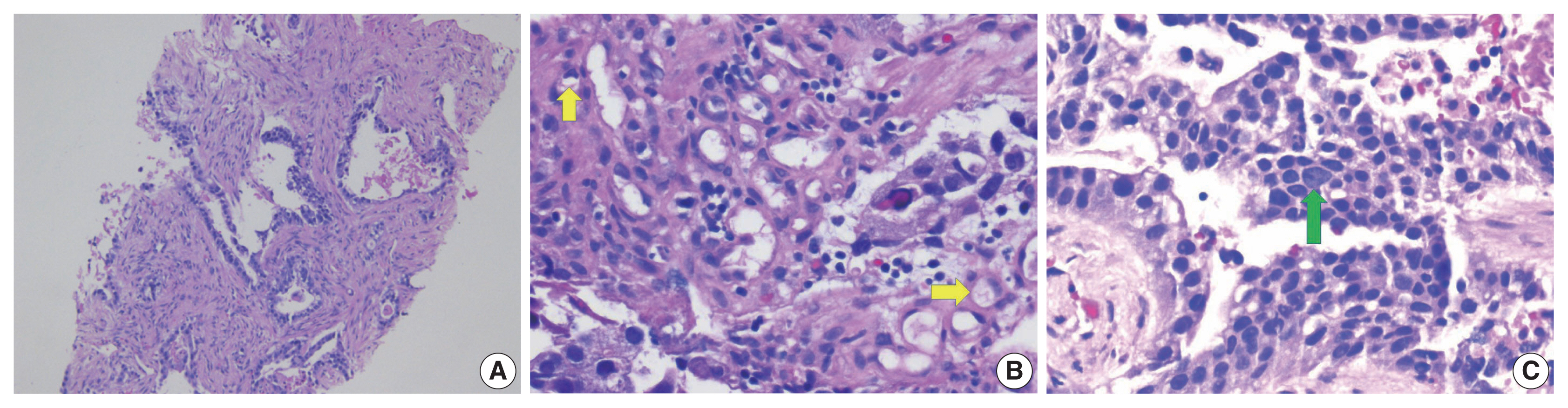
- Colorectal adenocarcinoma with enteroblastic differentiation (CAED) is a rare subtype of colonic adenocarcinoma characterized by increased α-fetoprotein (AFP) production and the expression of at least one enteroblastic marker including AFP, glypican 3 (GPC3), or Spalt like transcription factor 4 (SALL4). We report a case of a 26-year-old female who presented with low back pain and constipation which persisted despite supportive measures. Imaging revealed multiple liver lesions and enlarged retroperitoneal nodes. Tumor markers including AFP were markedly elevated. On biopsy, samples from the liver revealed infiltrating glands lined by columnar-type epithelium with mostly eosinophilic granular to focally clear cytoplasm. By immunohistochemistry, the tumor showed immunoreactivity with AFP, hepatocyte antigen, GPC3, SALL4, CDX2, SATB2, and cytokeratin 20. A colonoscopy performed subsequently revealed a mass in the sigmoid colon and biopsy of this mass revealed a similar histology as that seen in the liver. A diagnosis of CAED was made, following the results of gene expression profiling by the tumor with next-generation sequencing which identified pathogenic variants in MUTYH, TP53, and KDM6A genes and therefore supported its colonic origin. Cases such as this underscores the use of ancillary diagnostic techniques in arriving at the correct diagnosis in lesions with overlapping clinicopathologic characteristics.
Keyword
Figure
Reference
-
References
1. Yamashiro Y, Saito T, Hayashi T, et al. Molecular and clinicopathological features of colorectal adenocarcinoma with enteroblastic differentiation. Histopathology. 2020; 77:492–502.
Article2. Murakami T, Yao T, Mitomi H, et al. Clinicopathologic and immunohistochemical characteristics of gastric adenocarcinoma with enteroblastic differentiation: a study of 29 cases. Gastric Cancer. 2016; 19:498–507.
Article3. Liu X, Sheng W, Wang Y. An analysis of clinicopathological features and prognosis by comparing hepatoid adenocarcinoma of the stomach with AFP-producing gastric cancer. J Surg Oncol. 2012; 106:299–303.
Article4. Anzai H, Kazama S, Kiyomatsu T, et al. Alpha-fetoprotein-producing early rectal carcinoma: a rare case report and review. World J Surg Oncol. 2015; 13:180.
Article5. Ren F, Weng W, Zhang Q, et al. Clinicopathological features and prognosis of AFP-producing colorectal cancer: a single-center analysis of 20 cases. Cancer Manag Res. 2019; 11:4557–67.6. Bergstrand CG, Czar B. Demonstration of a new protein fraction in serum from the human fetus. Scand J Clin Lab Invest. 1956; 8:174.
Article7. O’Conor GT, Tatarinov YS, Abelev GI, Uriel J. A collaborative study for the evaluation of a serologic test for primary liver cancer. Cancer. 1970; 25:1091–8.
Article8. Bae JM, Lee TH, Cho NY, Kim TY, Kang GH. Loss of CDX2 expression is associated with poor prognosis in colorectal cancer patients. World J Gastroenterol. 2015; 21:1457–67.
Article9. McIntire KR, Waldmann TA, Moertel CG, Go VL. Serum alpha-fetoprotein in patients with neoplasms of the gastrointestinal tract. Cancer Res. 1975; 35:991–6.10. Nakajima T, Okazaki N, Morinaga S, Tsumuraya M, Shimosato Y, Saiki S. A case of alpha-fetoprotein-producing rectal carcinoma. Jpn J Clin Oncol. 1985; 15:679–85.11. Furuya Y, Wakahara T, Akimoto H, et al. Clear cell adenocarcinoma with enteroblastic differentiation of the ascending colon. J Clin Oncol. 2011; 29:e647–9.
Article12. Murakami T, Yao T, Yatagai N, et al. Colorectal adenocarcinoma with enteroblastic differentiation: a clinicopathological study of five cases. Histopathology. 2020; 76:325–32.
Article13. Ogiwara S, Furihata M, Fukami K, Yamashita A, Yao T, Osada T. Hepatoid adenocarcinoma with enteroblastic differentiation in the sigmoid colon: lessons from a rare case. Am J Gastroenterol. 2019; 114:684–5.
Article14. Eloy C, Lopes JM, Faria G, et al. Clear cell change in colonic polyps. Int J Surg Pathol. 2009; 17:438–43.
Article15. Matsunou H, Konishi F, Jalal RE, Yamamichi N, Mukawa A. Alpha-fetoprotein-producing gastric carcinoma with enteroblastic differentiation. Cancer. 1994; 73:534–40.
Article16. Yasuda K, Inomata M, Shiromizu A, Shiraishi N, Higashi H, Kitano S. Risk factors for occult lymph node metastasis of colorectal cancer invading the submucosa and indications for endoscopic mucosal resection. Dis Colon Rectum. 2007; 50:1370–6.
Article17. Nojadeh JN, Behrouz Sharif S, Sakhinia E. Microsatellite instability in colorectal cancer. EXCLI J. 2018; 17:159–68.18. Iacopetta B. TP53 mutation in colorectal cancer. Hum Mutat. 2003; 21:271–6.19. Li XL, Zhou J, Chen ZR, Chng WJ. P53 mutations in colorectal cancer: molecular pathogenesis and pharmacological reactivation. World J Gastroenterol. 2015; 21:84–93.
Article20. Natsume S, Yamaguchi T, Takao M, et al. Clinicopathological and molecular differences between right-sided and left-sided colorectal cancer in Japanese patients. Jpn J Clin Oncol. 2018; 48:609–18.
Article21. Nakamura Y, Matsuda K, Yokoyama S, et al. Alpha-fetoprotein-producing rectal cancer successfully responded to preoperative chemoradiotherapy: case report. Surg Case Rep. 2018; 4:111.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Gastric Cancer Showing Rapid Recurrence and Progression: A Case of Gastric Adenocarcinoma With Enteroblastic Differentiation
- Gastric adenocarcinoma with enteroblastic differentiation in a 67-year-old man in Korea: a case report
- A Case of Synchronous Colorectal Adenocarcinoma with Anal Squamous Cell Carcinoma
- Endometrial Mucinous Adenocarcinoma with Extensive Squamous Differentiation: A Case Report
- Gastric-Type Extremely Well-Differentiated Adenocarcinoma of the Stomach: A Challenge for Preoperative Diagnosis