Int J Stem Cells.
2022 Feb;15(1):85-94. 10.15283/ijsc21157.
Generation of Cortical Brain Organoid with Vascularization by Assembling with Vascular Spheroid
- Affiliations
-
- 1Adult Stem Cell Research Center, College of Veterinary Medicine, Seoul National University, Seoul, Korea
- 2College of Veterinary Medicine and Research Institute for Veterinary Science, Seoul National University, Seoul, Korea
- KMID: 2526740
- DOI: http://doi.org/10.15283/ijsc21157
Abstract
- Background and Objectives
Brain organoids have the potential to improve our understanding of brain development and neurological disease. Despite the importance of brain organoids, the effect of vascularization on brain organoids is largely unknown. The objective of this study is to develop vascularized organoids by assembling vascular spheroids with cerebral organoids.
Methods and Results
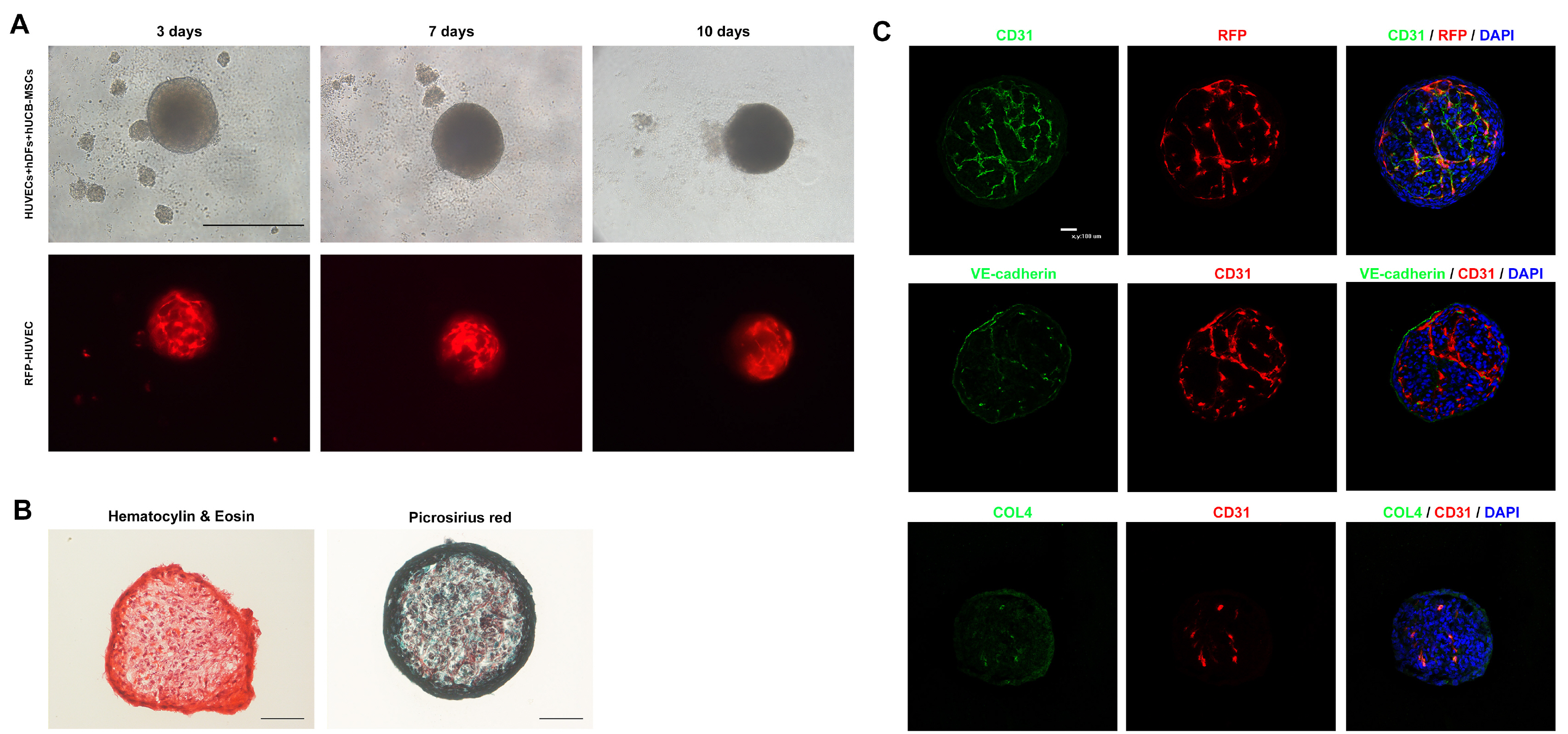
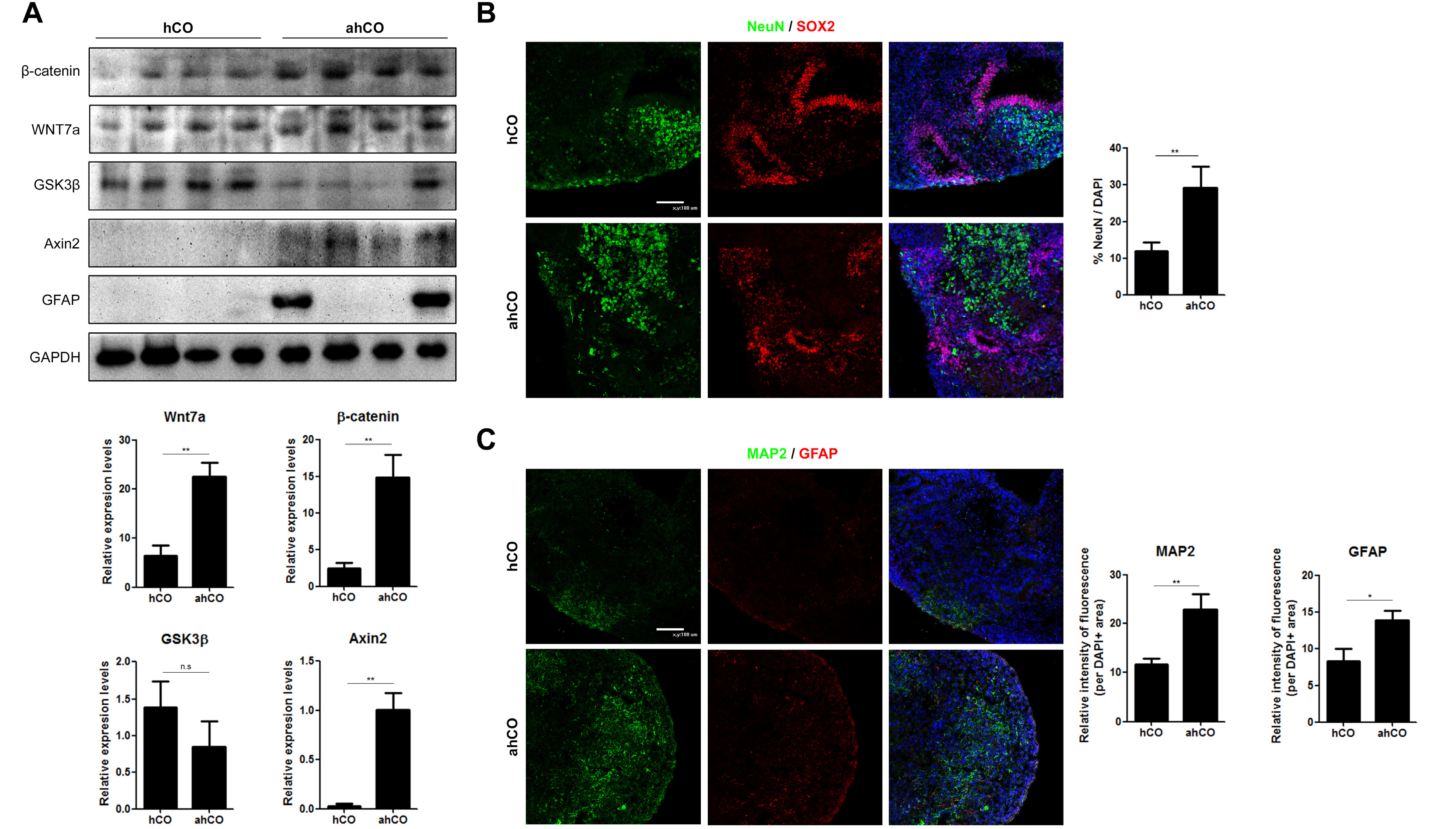
In this study, vascularized spheroids were generated from non-adherent microwell culture system of human umbilical vein endothelial cells, human dermal fibroblasts and human umbilical cord blood derived mesenchymal stem cells. These vascular spheroids were used for fusion with iPSCs induced cerebral organoids. Immunostaining studies of vascularized organoids demonstrated well organized vascular structures and reduced apoptosis. We showed that the vascularization in cerebral organoids up-regulated the Wnt/β-catenin signaling.
Conclusions
We developed vascularized cerebral organoids through assembly of brain organoids with vascular spheroids. This method could not only provide a model to study human cortical development but also represent an opportunity to explore neurological disease.
Keyword
Figure
Cited by 1 articles
-
Lo and Behold, the Lab-Grown Organs Have Arrived!
Jaesang Kim
Int J Stem Cells. 2022;15(1):1-2. doi: 10.15283/ijsc22026.
Reference
-
References
1. Marshall JJ, Mason JO. 2019; Mouse vs man: organoid models of brain development & disease. Brain Res. 1724:146427. DOI: 10.1016/j.brainres.2019.146427. PMID: 31473222.2. Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, Knoblich JA. 2013; Cerebral organoids model human brain development and microcephaly. Nature. 501:373–379. DOI: 10.1038/nature12517. PMID: 23995685. PMCID: PMC3817409.
Article3. Lancaster MA, Knoblich JA. 2014; Generation of cerebral organoids from human pluripotent stem cells. Nat Protoc. 9:2329–2340. DOI: 10.1038/nprot.2014.158. PMID: 25188634. PMCID: PMC4160653.
Article4. Qian X, Song H, Ming GL. 2019; Brain organoids: advances, applications and challenges. Development. 146:dev166074. DOI: 10.1242/dev.166074. PMID: 30992274. PMCID: PMC6503989.
Article5. Clevers H. 2016; Modeling development and disease with organoids. Cell. 165:1586–1597. DOI: 10.1016/j.cell.2016.05.082. PMID: 27315476.
Article6. Luo C, Lancaster MA, Castanon R, Nery JR, Knoblich JA, Ecker JR. 2016; Cerebral organoids recapitulate epigenomic signatures of the human fetal brain. Cell Rep. 17:3369–3384. DOI: 10.1016/j.celrep.2016.12.001. PMID: 28009303. PMCID: PMC5495578.
Article7. Sloan SA, Andersen J, Pașca AM, Birey F, Pașca SP. 2018; Generation and assembly of human brain region-specific three-dimensional cultures. Nat Protoc. 13:2062–2085. DOI: 10.1038/s41596-018-0032-7. PMID: 30202107. PMCID: PMC6597009.
Article8. Koo B, Choi B, Park H, Yoon KJ. 2019; Past, present, and future of brain organoid technology. Mol Cells. 42:617–627. DOI: 10.14348/molcells.2019.0162. PMID: 31564073. PMCID: PMC6776157.9. Matsui TK, Tsuru Y, Hasegawa K, Kuwako KI. 2021; Vasculari-zation of human brain organoids. Stem Cells. 39:1017–1024. DOI: 10.1002/stem.3368. PMID: 33754425.
Article10. Cakir B, Xiang Y, Tanaka Y, Kural MH, Parent M, Kang YJ, Chapeton K, Patterson B, Yuan Y, He CS, Raredon MSB, Dengelegi J, Kim KY, Sun P, Zhong M, Lee S, Patra P, Hyder F, Niklason LE, Lee SH, Yoon YS, Park IH. 2019; Engineering of human brain organoids with a functional vascular-like system. Nat Methods. 16:1169–1175. DOI: 10.1038/s41592-019-0586-5. PMID: 31591580. PMCID: PMC6918722.
Article11. Shi Y, Sun L, Wang M, Liu J, Zhong S, Li R, Li P, Guo L, Fang A, Chen R, Ge WP, Wu Q, Wang X. 2020; Vascularized human cortical organoids (vOrganoids) model cortical development in vivo. PLoS Biol. 18:e3000705. DOI: 10.1371/journal.pbio.3000705. PMID: 32401820. PMCID: PMC7250475. PMID: 66f6d290c2f84ede886185d58cd7368f.12. Licht T, Keshet E. 2015; The vascular niche in adult neurogenesis. Mech Dev. 138 Pt 1:56–62. DOI: 10.1016/j.mod.2015.06.001. PMID: 26103548.
Article13. Karakatsani A, Shah B, Ruiz de Almodovar C. 2019; Blood vessels as regulators of neural stem cell properties. Front Mol Neurosci. 12:85. DOI: 10.3389/fnmol.2019.00085. PMID: 31031591. PMCID: PMC6473036.
Article14. Daneman R, Agalliu D, Zhou L, Kuhnert F, Kuo CJ, Barres BA. 2009; Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc Natl Acad Sci U S A. 106:641–646. DOI: 10.1073/pnas.0805165106. PMID: 19129494. PMCID: PMC2626756.15. Chenn A. 2008; Wnt/beta-catenin signaling in cerebral cortical development. Organogenesis. 4:76–80. DOI: 10.4161/org.4.2.5852. PMID: 19279718. PMCID: PMC2634251.
Article16. De Moor L, Merovci I, Baetens S, Verstraeten J, Kowalska P, Krysko DV, De Vos WH, Declercq H. 2018; High-throughput fabrication of vascularized spheroids for bioprinting. Biofabrication. 10:035009. DOI: 10.1088/1758-5090/aac7e6. PMID: 29798932.
Article17. Mulligan KA, Cheyette BN. 2012; Wnt signaling in vertebrate neural development and function. J Neuroimmune Phar-macol. 7:774–787. DOI: 10.1007/s11481-012-9404-x. PMID: 23015196. PMCID: PMC3518582.
Article18. Muoio V, Persson PB, Sendeski MM. 2014; The neurovascular unit - concept review. Acta Physiol (Oxf). 210:790–798. DOI: 10.1111/apha.12250. PMID: 24629161.
Article19. Bell AH, Miller SL, Castillo-Melendez M, Malhotra A. 2020; The neurovascular unit: effects of brain insults during the perinatal period. Front Neurosci. 13:1452. DOI: 10.3389/fnins.2019.01452. PMID: 32038147. PMCID: PMC6987380. PMID: 469ce704fd7343508e166500e1437879.
Article20. Pham MT, Pollock KM, Rose MD, Cary WA, Stewart HR, Zhou P, Nolta JA, Waldau B. 2018; Generation of human vascularized brain organoids. Neuroreport. 29:588–593. DOI: 10.1097/WNR.0000000000001014. PMID: 29570159. PMCID: PMC6476536.
Article21. Zhao X, Xu Z, Xiao L, Shi T, Xiao H, Wang Y, Li Y, Xue F, Zeng W. 2021; Review on the vascularization of organoids and organoids-on-a-chip. Front Bioeng Biotechnol. 9:637048. DOI: 10.3389/fbioe.2021.637048. PMID: 33912545. PMCID: PMC8072266. PMID: d9603ef2f6584acfa5016be2d649b8c3.
Article22. Rademakers T, Horvath JM, van Blitterswijk CA, LaPointe VLS. 2019; Oxygen and nutrient delivery in tissue engineering: approaches to graft vascularization. J Tissue Eng Regen Med. 13:1815–1829. DOI: 10.1002/term.2932. PMID: 31310055. PMCID: PMC6852121.
Article23. Heiss M, Hellström M, Kalén M, May T, Weber H, Hecker M, Augustin HG, Korff T. 2015; Endothelial cell spheroids as a versatile tool to study angiogenesis in vitro. FASEB J. 29:3076–3084. DOI: 10.1096/fj.14-267633. PMID: 25857554.
Article24. Pfisterer L, Korff T. 2016; Spheroid-based in vitro angiogenesis model. Methods Mol Biol. 1430:167–177. DOI: 10.1007/978-1-4939-3628-1_11. PMID: 27172953.25. Serra J, Alves CPA, Brito L, Monteiro GA, Cabral JMS, Prazeres DMF, da Silva CL. 2019; Engineering of human mesenchymal stem/stromal cells with vascular endothelial growth factor-encoding minicircles for angiogenic ex vivo gene therapy. Hum Gene Ther. 30:316–329. DOI: 10.1089/hum.2018.154. PMID: 30200778.
Article26. Arutyunyan I, Fatkhudinov T, Kananykhina E, Usman N, Elchaninov A, Makarov A, Bolshakova G, Goldshtein D, Sukhikh G. 2016; Role of VEGF-A in angiogenesis promoted by umbilical cord-derived mesenchymal stromal/stem cells: in vitro study. Stem Cell Res Ther. 7:46. DOI: 10.1186/s13287-016-0305-4. PMID: 27001300. PMCID: PMC4802928.27. Maacha S, Sidahmed H, Jacob S, Gentilcore G, Calzone R, Grivel JC, Cugno C. 2020; Paracrine mechanisms of mesenchymal stromal cells in angiogenesis. Stem Cells Int. 2020:4356359. DOI: 10.1155/2020/4356359. PMID: 32215017. PMCID: PMC7085399.
Article28. Mansour AA, Gonçalves JT, Bloyd CW, Li H, Fernandes S, Quang D, Johnston S, Parylak SL, Jin X, Gage FH. 2018; An in vivo model of functional and vascularized human brain organoids. Nat Biotechnol. 36:432–441. DOI: 10.1038/nbt.4127. PMID: 29658944. PMCID: PMC6331203.
Article29. Lau M, Li J, Cline HT. 2017; In vivo analysis of the neurovascular niche in the developing Xenopus brain. eNeuro. 4:ENEURO.0030-17.2017. DOI: 10.1523/ENEURO.0030-17.2017. PMID: 28795134. PMCID: PMC5548361.30. Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, Garcia-Verdugo JM, Doetsch F. 2008; A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 3:279–288. DOI: 10.1016/j.stem.2008.07.025. PMID: 18786415. PMCID: PMC6864413.
Article31. Tang M, Gao G, Rueda CB, Yu H, Thibodeaux DN, Awano T, Engelstad KM, Sanchez-Quintero MJ, Yang H, Li F, Li H, Su Q, Shetler KE, Jones L, Seo R, McConathy J, Hillman EM, Noebels JL, De Vivo DC, Monani UR. 2017; Brain microvasculature defects and Glut1 deficiency syndrome averted by early repletion of the glucose transporter-1 protein. Nat Commun. 8:14152. DOI: 10.1038/ncomms14152. PMID: 28106060. PMCID: PMC5263887. PMID: cfe083483d664856ac5c4ced1381ccce.
Article32. Tang M, Park SH, Petri S, Yu H, Rueda CB, Abel ED, Kim CY, Hillman EM, Li F, Lee Y, Ding L, Jagadish S, Frankel WN, De Vivo DC, Monani UR. 2021; An early endothelial cell-specific requirement for Glut1 is revealed in Glut1 deficiency syndrome model mice. JCI Insight. 6:e145789. DOI: 10.1172/jci.insight.145789. PMID: 33351789. PMCID: PMC7934852.
Article33. Wexler EM, Paucer A, Kornblum HI, Palmer TD, Geschwind DH. 2009; Endogenous Wnt signaling maintains neural progenitor cell potency. Stem Cells. 27:1130–1141. DOI: 10.1002/stem.36. PMID: 19418460. PMCID: PMC2782960.
Article34. Kriska J, Janeckova L, Kirdajova D, Honsa P, Knotek T, Dzamba D, Kolenicova D, Butenko O, Vojtechova M, Capek M, Kozmik Z, Taketo MM, Korinek V, Anderova M. 2021; Wnt/β-catenin signaling promotes differentiation of ischemia-activated adult neural stem/progenitor cells to neuronal precursors. Front Neurosci. 15:628983. DOI: 10.3389/fnins.2021.628983. PMID: 33716653. PMCID: PMC7947698. PMID: abcd8bb230064c7b80ad86ff6baa7608.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Simple Method for Generating Cerebral Organoids from Human Pluripotent Stem Cells
- In Vitro Generation of Luminal Vasculature in Liver Organoids: From Basic Vascular Biology to Vascularized Hepatic Organoids
- Engineering Human Brain Organoids: From Basic Research to Tissue Regeneration
- Mesenchymal Stem Cell Spheroids: A Promising Tool for Vascularized Tissue Regeneration
- Increased SOX2 expression in three-dimensional sphere culture of dental pulp stem cells