Korean J Physiol Pharmacol.
2022 Mar;26(2):125-133. 10.4196/kjpp.2022.26.2.125.
Carbon monoxide releasing molecule-2 suppresses stretchactivated atrial natriuretic peptide secretion by activating largeconductance calcium-activated potassium channels
- Affiliations
-
- 1Departments of Physiology, Jeonbuk National University Medical School, Jeonju 54907, Korea
- 2Departments of Internal Medicine, Jeonbuk National University Medical School, Jeonju 54907, Korea
- 3Research Institute of Clinical Medicine of Jeonbuk National University Jeonju 54907, Korea
- KMID: 2526731
- DOI: http://doi.org/10.4196/kjpp.2022.26.2.125
Abstract
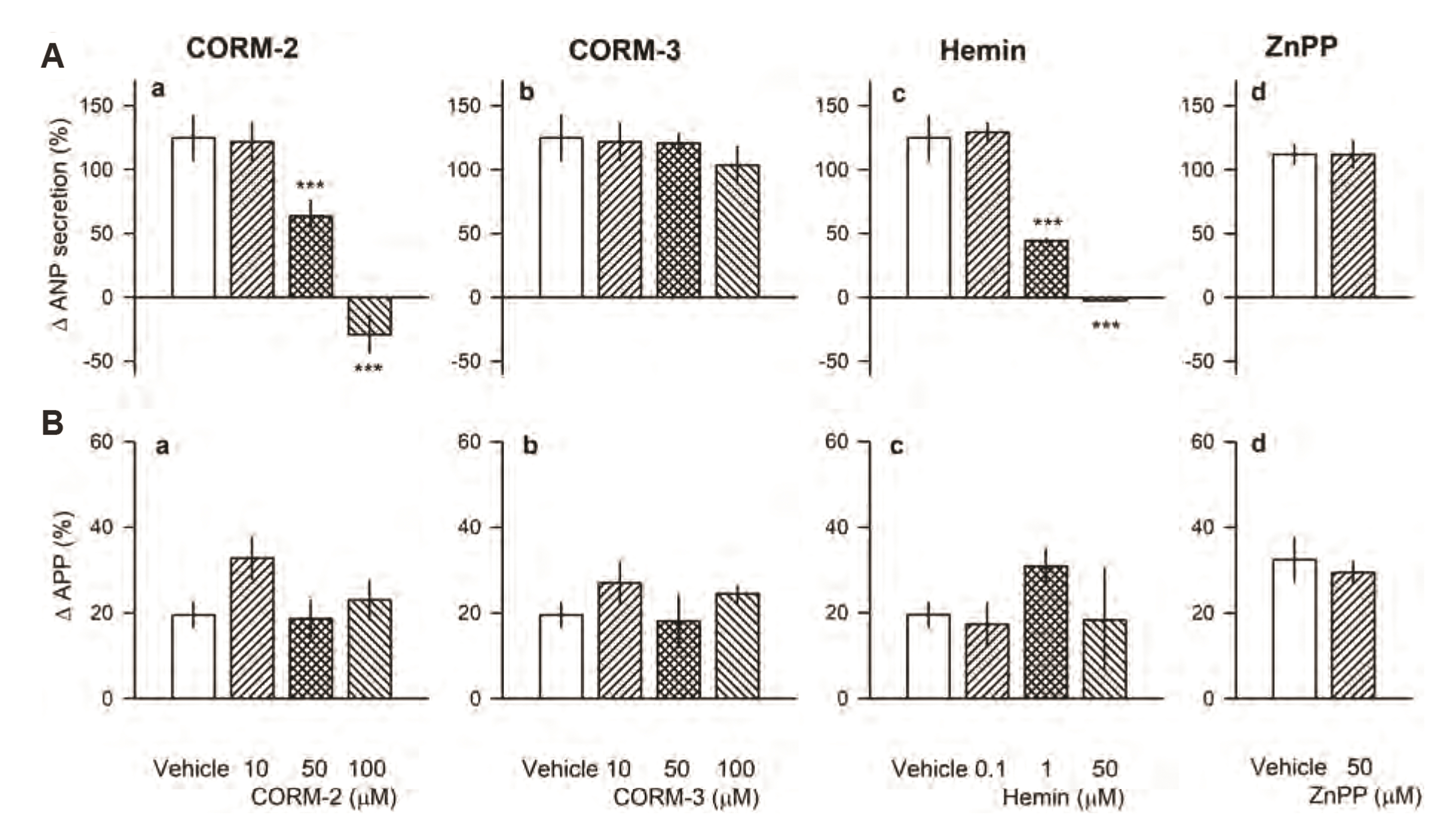
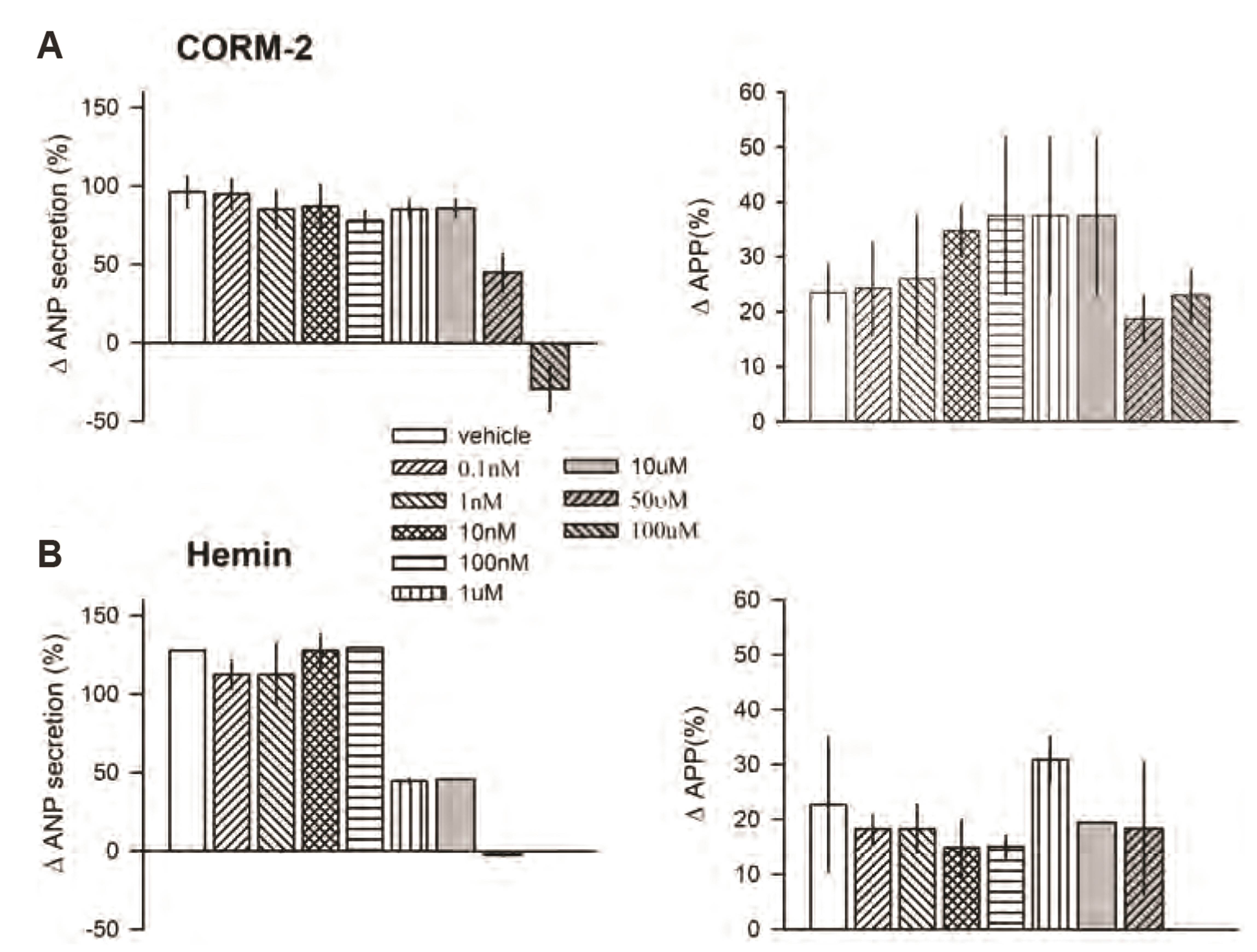
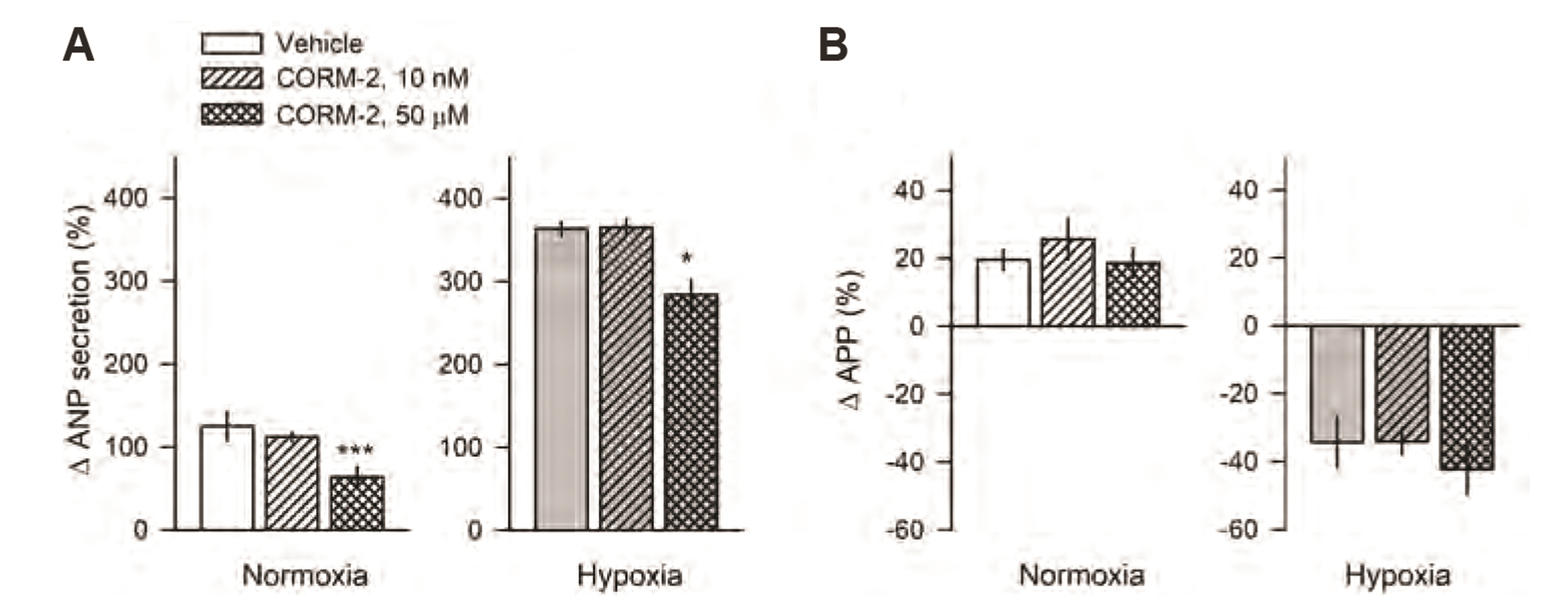
- Carbon monoxide (CO) is a known gaseous bioactive substance found across a wide array of body systems. The administration of low concentrations of CO has been found to exert an anti-inflammatory, anti-apoptotic, anti-hypertensive, and vaso-dilatory effect. To date, however, it has remained unknown whether CO influences atrial natriuretic peptide (ANP) secretion. This study explores the effect of CO on ANP secretion and its associated signaling pathway using isolated beating rat atria. Atrial perfusate was collected for 10 min for use as a control, after which high atrial stretch was induced by increasing the height of the outflow catheter. Carbon monoxide releasing molecule-2 (CORM-2; 10, 50, 100 µM) and hemin (HO-1 inducer; 0.1, 1, 50 µM), but not CORM-3 (10, 50, 100 µM), decreased high stretch-induced ANP secretion. However, zinc porphyrin (HO-1 inhibitor) did not affect ANP secretion. The order of potency for the suppression of ANP secretion was found to be hemin > CORM-2 >> CORM-3. The suppression of ANP secretion by CORM-2 was attenuated by pretreatment with 5-hydroxydecanoic acid, paxilline, and 1H-[1,2,4] oxadiazolo [4,3-a] quinoxalin-1-one, but not by diltiazem, wortmannin, LY-294002, or NG-nitroL-arginine methyl ester. Hypoxic conditions attenuated the suppressive effect of CORM-2 on ANP secretion. In sum, these results suggest that CORM-2 suppresses ANP secretion via mitochondrial K ATP channels and large conductance Ca 2+ -activated K+ channels.
Keyword
Figure
Reference
-
1. Tenhunen R, Marver HS, Schmid R. 1968; The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci U S A. 61:748–755. DOI: 10.1073/pnas.61.2.748. PMID: 4386763. PMCID: PMC225223.
Article2. Szabo C. 2010; Gaseotransmitters: new frontiers for translational science. Sci Transl Med. 2:59ps54. DOI: 10.1126/scitranslmed.3000721. PMID: 21106939. PMCID: PMC3038605.
Article3. Caliendo G, Cirino G, Santagada V, Wallace JL. 2010; Synthesis and biological effects of hydrogen sulfide (H2S): development of H2S-releasing drugs as pharmaceuticals. J Med Chem. 53:6275–6286. DOI: 10.1021/jm901638j. PMID: 20462257.
Article4. Peers C, Steele DS. 2012; Carbon monoxide: a vital signalling molecule and potent toxin in the myocardium. J Mol Cell Cardiol. 52:359–365. DOI: 10.1016/j.yjmcc.2011.05.013. PMID: 21640728.
Article5. Kim HP, Ryter SW, Choi AM. 2006; CO as a cellular signaling molecule. Annu Rev Pharmacol Toxicol. 46:411–449. DOI: 10.1146/annurev.pharmtox.46.120604.141053. PMID: 16402911.
Article6. Andreadou I, Iliodromitis EK, Rassaf T, Schulz R, Papapetropoulos A, Ferdinandy P. 2015; The role of gasotransmitters NO, H2S and CO in myocardial ischaemia/reperfusion injury and cardioprotection by preconditioning, postconditioning and remote conditioning. Br J Pharmacol. 172:1587–1606. DOI: 10.1111/bph.12811. PMID: 24923364. PMCID: PMC4369266.
Article7. Haldane J. 1895; The relation of the action of carbonic oxide to oxygen tension. J Physiol. 18:201–217. DOI: 10.1113/jphysiol.1895.sp000562. PMID: 16992250. PMCID: PMC1514632.
Article8. André L, Gouzi F, Thireau J, Meyer G, Boissiere J, Delage M, Abdellaoui A, Feillet-Coudray C, Fouret G, Cristol JP, Lacampagne A, Obert P, Reboul C, Fauconnier J, Hayot M, Richard S, Cazorla O. 2011; Carbon monoxide exposure enhances arrhythmia after cardiac stress: involvement of oxidative stress. Basic Res Cardiol. 106:1235–1246. DOI: 10.1007/s00395-011-0211-y. PMID: 21822772.
Article9. Dallas ML, Yang Z, Boyle JP, Boycott HE, Scragg JL, Milligan CJ, Elies J, Duke A, Thireau J, Reboul C, Richard S, Bernus O, Steele DS, Peers C. 2012; Carbon monoxide induces cardiac arrhythmia via induction of the late Na+ current. Am J Respir Crit Care Med. 186:648–656. DOI: 10.1164/rccm.201204-0688OC. PMID: 22822026. PMCID: PMC3622900.
Article10. Gandini C, Castoldi AF, Candura SM, Locatelli C, Butera R, Priori S, Manzo L. 2001; Carbon monoxide cardiotoxicity. J Toxicol Clin Toxicol. 39:35–44. DOI: 10.1081/CLT-100102878. PMID: 11327225.
Article11. Ryter SW, Otterbein LE. 2004; Carbon monoxide in biology and medicine. Bioessays. 26:270–280. DOI: 10.1002/bies.20005. PMID: 14988928.
Article12. Stein AB, Guo Y, Tan W, Wu WJ, Zhu X, Li Q, Luo C, Dawn B, Johnson TR, Motterlini R, Bolli R. 2005; Administration of a CO-releasing molecule induces late preconditioning against myocardial infarction. J Mol Cell Cardiol. 38:127–134. DOI: 10.1016/j.yjmcc.2004.10.006. PMID: 15623429. PMCID: PMC3199606.
Article13. Muchova L, Wong RJ, Hsu M, Morioka I, Vitek L, Zelenka J, Schröder H, Stevenson DK. 2007; Statin treatment increases formation of carbon monoxide and bilirubin in mice: a novel mechanism of in vivo antioxidant protection. Can J Physiol Pharmacol. 85:800–810. DOI: 10.1139/Y07-077. PMID: 17901890.
Article14. Lakkisto P, Siren JM, Kytö V, Forsten H, Laine M, Pulkki K, Tikkanen I. 2011; Heme oxygenase-1 induction protects the heart and modulates cellular and extracellular remodelling after myocardial infarction in rats. Exp Biol Med (Maywood). 236:1437–1448. DOI: 10.1258/ebm.2011.011148. PMID: 22087023.
Article15. Ewing JF, Raju VS, Maines MD. 1994; Induction of heart heme oxygenase-1 (HSP32) by hyperthermia: possible role in stress-mediated elevation of cyclic 3':5'-guanosine monophosphate. J Pharmacol Exp Ther. 271:408–414. PMID: 7525927.16. Foresti R, Goatly H, Green CJ, Motterlini R. 2001; Role of heme oxygenase-1 in hypoxia-reoxygenation: requirement of substrate heme to promote cardioprotection. Am J Physiol Heart Circ Physiol. 281:H1976–H1984. DOI: 10.1152/ajpheart.2001.281.5.H1976. PMID: 11668058.
Article17. Stec DE, Drummond HA, Vera T. 2008; Role of carbon monoxide in blood pressure regulation. Hypertension. 51:597–604. DOI: 10.1161/HYPERTENSIONAHA.107.097154. PMID: 18212274.
Article18. de Bold AJ, Borenstein HB, Veress AT, Sonnenberg H. 1981; A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Life Sci. 28:89–94. DOI: 10.1016/0024-3205(81)90370-2. PMID: 7219045.
Article19. de Bold AJ. 2011; Thirty years of research on atrial natriuretic factor: historical background and emerging concepts. Can J Physiol Pharmacol. 89:527–531. DOI: 10.1139/y11-019. PMID: 21671768.
Article20. Goetze JP, Bruneau BG, Ramos HR, Ogawa T, de Bold MK, de Bold AJ. 2020; Cardiac natriuretic peptides. Nat Rev Cardiol. 17:698–717. DOI: 10.1038/s41569-020-0381-0. PMID: 32444692.
Article21. Cho KW, Seul KH, Ryu H, Kim SH, Koh GY. 1988; Characteristics of distension-induced release of immunoreactive atrial natriuretic peptide in isolated perfused rabbit atria. Regul Pept. 22:333–345. DOI: 10.1016/0167-0115(88)90110-3. PMID: 2973090.
Article22. Zimmerman RS, Ryan J, Edwards BS, Klee G, Zimmerman D, Scott N, Burnett JC Jr. 1988; Cardiorenal endocrine dynamics during volume expansion in hypothyroid dogs. Am J Physiol. 255(1 Pt 2):R61–R66. DOI: 10.1152/ajpregu.1988.255.1.R61. PMID: 2969197.
Article23. Skvorak JP, Nazian SJ, Dietz JR. 1995; Endothelin acts as a paracrine regulator of stretch-induced atrial natriuretic peptide release. Am J Physiol. 269(5 Pt 2):R1093–R1098. DOI: 10.1152/ajpregu.1995.269.5.R1093. PMID: 7503296.
Article24. Dietz JR. 2005; Mechanisms of atrial natriuretic peptide secretion from the atrium. Cardiovasc Res. 68:8–17. DOI: 10.1016/j.cardiores.2005.06.008. PMID: 15993390.
Article25. Varadi J, Lekli I, Juhasz B, Bacskay I, Szabo G, Gesztelyi R, Szendrei L, Varga E, Bak I, Foresti R, Motterlini R, Tosaki A. 2007; Beneficial effects of carbon monoxide-releasing molecules on post-ischemic myocardial recovery. Life Sci. 80:1619–1626. DOI: 10.1016/j.lfs.2007.01.047. PMID: 17321552.
Article26. Motterlini R. 2007; Carbon monoxide-releasing molecules (CO-RMs): vasodilatory, anti-ischaemic and anti-inflammatory activities. Biochem Soc Trans. 35(Pt 5):1142–1146. DOI: 10.1042/BST0351142. PMID: 17956297.
Article27. Yuan K, Cao C, Han JH, Kim SZ, Kim SH. 2005; Adenosine-stimulated atrial natriuretic peptide release through A1 receptor subtype. Hypertension. 46:1381–1387. DOI: 10.1161/01.HYP.0000190041.61737.fd. PMID: 16286581.
Article28. Han JH, Cao C, Kim SZ, Cho KW, Kim SH. 2003; Decreases in ANP secretion by lysophosphatidylcholine through protein kinase C. Hypertension. 41:1380–1385. DOI: 10.1161/01.HYP.0000071317.98004.B3. PMID: 12719444.
Article29. Cho KW, Kim SH, Koh GY, Seul KH, Huh KS, Chu D, Rapp NS, Moon HB, Kim KK, Kook YJ. 1989; Plasma concentration of atrial natriuretic peptide in different phases of Korean hemorrhagic fever. Nephron. 51:215–219. DOI: 10.1159/000185288. PMID: 2563575.
Article30. Cho KW, Kim SH, Kim CH, Seul KH. 1995; Mechanical basis of ANP secretion in beating atria: atrial stroke volume and ECF translocation. Am J Physiol. 268(5 Pt 2):R1129–R1136. DOI: 10.1152/ajpregu.1995.268.5.R1129. PMID: 7771572.
Article31. Cho KW, Kim SH, Hwang YH, Seul KH. 1993; Extracellular fluid translocation in perfused rabbit atria: implication in control of atrial natriuretic peptide secretion. J Physiol. 468:591–607. DOI: 10.1113/jphysiol.1993.sp019790. PMID: 8254526. PMCID: PMC1143845.
Article32. Rochetaing A, Barbé C, Kreher P. 2001; Acute ischemic preconditioning and high subchronic CO exposure independently increase myocardial tolerance to ischemia. Inhal Toxicol. 13:1015–1032. DOI: 10.1080/089583701753210380. PMID: 11696871.
Article33. Soni HM, Jain MR, Mehta AA. 2012; Mechanism(s) involved in carbon monoxide-releasing molecule-2-mediated cardioprotection during ischaemia-reperfusion injury in isolated rat heart. Indian J Pharm Sci. 74:281–291. DOI: 10.4103/0250-474X.107047. PMID: 23626383. PMCID: PMC3630723.
Article34. Clark JE, Naughton P, Shurey S, Green CJ, Johnson TR, Mann BE, Foresti R, Motterlini R. 2003; Cardioprotective actions by a water-soluble carbon monoxide-releasing molecule. Circ Res. 93:e2–e8. DOI: 10.1161/01.RES.0000084381.86567.08. PMID: 12842916.
Article35. Williams SE, Wootton P, Mason HS, Bould J, Iles DE, Riccardi D, Peers C, Kemp PJ. 2004; Hemoxygenase-2 is an oxygen sensor for a calcium-sensitive potassium channel. Science. 306:2093–2097. DOI: 10.1126/science.1105010. PMID: 15528406.
Article36. Riesco-Fagundo AM, Pérez-García MT, González C, López-López JR. 2001; O2 modulates large-conductance Ca2+-dependent K+ channels of rat chemoreceptor cells by a membrane-restricted and CO-sensitive mechanism. Circ Res. 89:430–436. DOI: 10.1161/hh1701.095632. PMID: 11532904.
Article37. Wang R, Wu L, Wang Z. 1997; The direct effect of carbon monoxide on KCa channels in vascular smooth muscle cells. Pflugers Arch. 434:285–291. DOI: 10.1007/s004240050398. PMID: 9178628.
Article38. Ryan MJ, Jernigan NL, Drummond HA, McLemore GR Jr, Rimoldi JM, Poreddy SR, Gadepalli RS, Stec DE. 2006; Renal vascular responses to CORM-A1 in the mouse. Pharmacol Res. 54:24–29. DOI: 10.1016/j.phrs.2006.01.012. PMID: 16524742.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Carbon monoxide activates large-conductance calcium-activated potassium channels of human cardiac fibroblasts through various mechanisms
- The WNT/Ca2+ pathway promotes atrial natriuretic peptide secretion by activating protein kinase C/transforming growth factor-β activated kinase 1/activating transcription factor 2 signaling in isolated beating rat atria
- A Clinical Observation of Acute Carbon Monoxide Poisoning
- Chorea as a Clinical Manifestation of Delayed Neurologic Sequelae in Carbon Monoxide Poisoning: Case Report
- A study of neurologic sequelae in carbon monoxide intoxication