Lab Med Online.
2021 Oct;11(4):267-274. 10.47429/lmo.2021.11.4.267.
Performance Evaluation of the CareGENETM Zika Virus Reverse Transcription-PCR Kit for Urine Specimen
- Affiliations
-
- 1Department of Laboratory Medicine, Korea University Guro Hospital, Seoul, Korea
- KMID: 2526088
- DOI: http://doi.org/10.47429/lmo.2021.11.4.267
Abstract
- Background
Zika virus (ZIKV) is a major health concern worldwide, highlighting the importance of accurate and reliable viral detection to control transmission. The careGENETM ZIKV Reverse Transcription-Polymerase Chain Reaction (RT-PCR) Kit (Wells Bio, INC, Korea) was developed to detect Zika infection. We compared the clinical sensitivity and specificity of this kit with those of the Aptima Zika Virus Assay (Hologic, Inc., USA) and the World Health Organization (WHO)-recommended conventional PCR method.
Methods
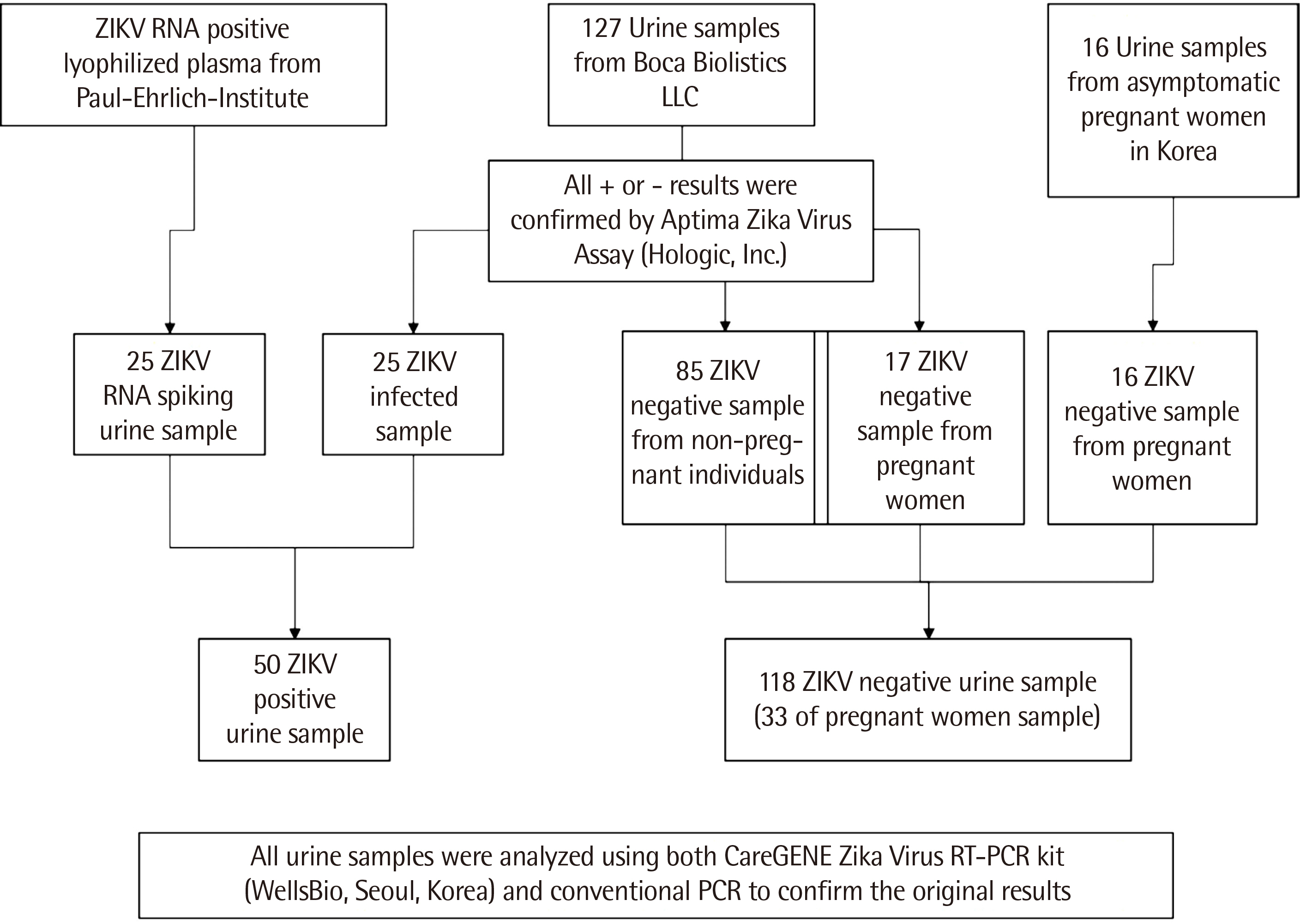
A total of 168 urine samples (143 clinical samples and 25 RNA-spiked samples [ RNA-positive lyophilized plasma spiked in urine ]) were tested in this study.
Results
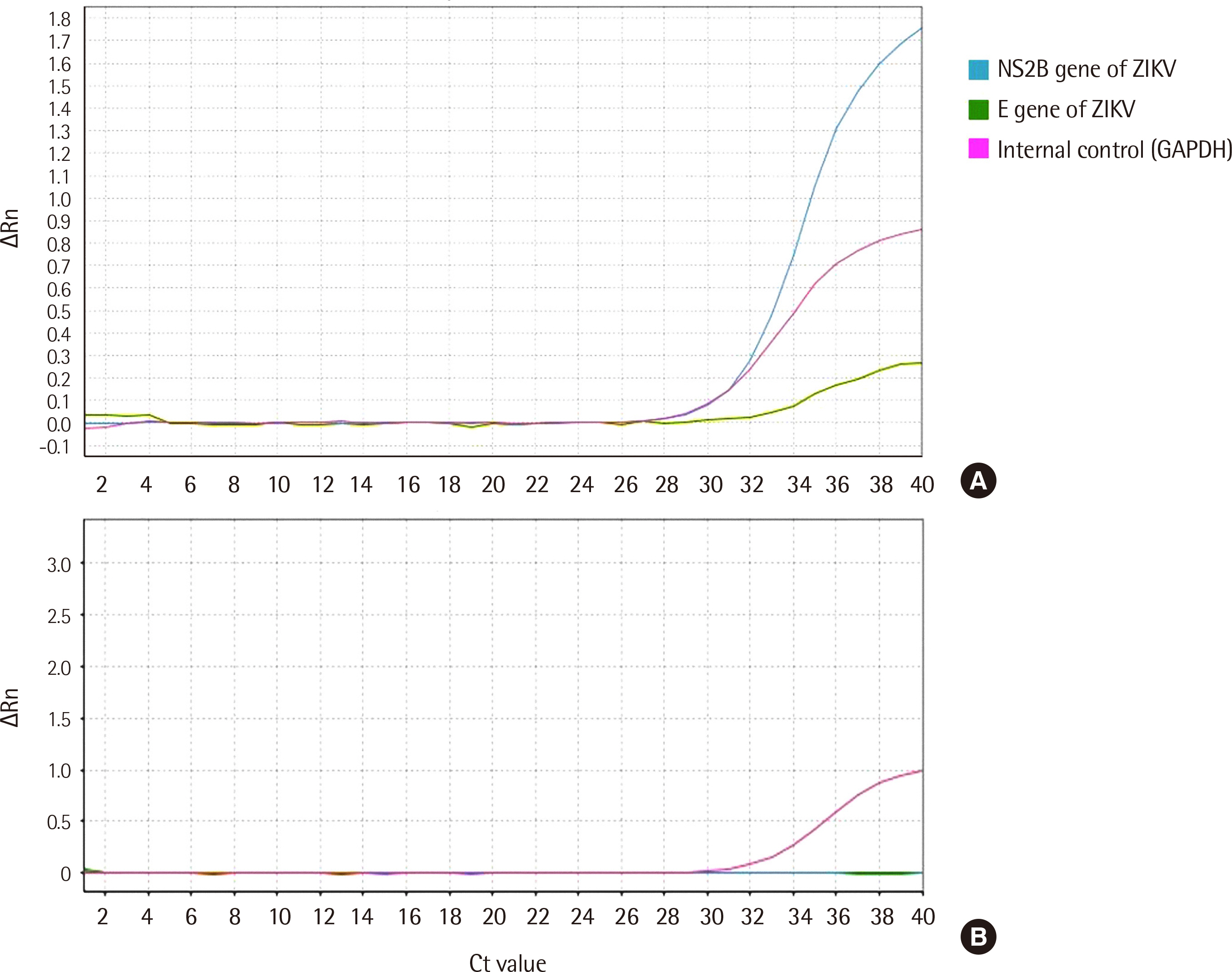
In comparison with the WHO-recommended conventional PCR method, the careGENETM ZIKV RT-PCR Kit detected ZIKV RNA with a sensitivity of 100% (95% confidence interval [ CI ], 73.5–99.9) and specificity of 100% (95% CI, 88.9–97.8). Similar results were obtained with the Aptima Zika Virus Assay, as evident from a sensitivity of 100% (95% CI, 73.5–99.9) and specificity of 100% (95% CI, 88.9–97.8). The limit of detection at a 95% detection probability was 1 U/mL in the urine.
Conclusions
Positive identification of Zika infection was selective and specific using the target Zika viral sequence. The overall performance of the careGENETM ZIKV RT-PCR Kit was satisfactory.
Figure
Reference
-
1. Dick GW, Kitchen SF, Haddow AJ. 1952; Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg. 46:509–20. DOI: 10.1016/0035-9203(52)90042-4. PMID: 12995440.
Article2. Waggoner JJ, Pinsky BA. 2016; Zika virus: diagnostics for an emerging pandemic threat. J Clin Microbiol. 54:860–7. DOI: 10.1128/JCM.00279-16. PMID: 26888897. PMCID: PMC4809954.
Article3. Campos GS, Bandeira AC, Sardi SI. 2015; Zika virus outbreak, Bahia, Brazil. Emerg Infect Dis. 21:1885–6. DOI: 10.3201/eid2110.150847. PMID: 26401719. PMCID: PMC4593454.
Article4. Jimenez A, Shaz BH, Kessler D, Bloch EM. 2017; How do we manage blood donors and recipients after a positive Zika screening result? Transfusion. 57:2077–83. DOI: 10.1111/trf.14252. PMID: 28734023.
Article5. Jang HC, Park WB, Kim UJ, Chun JY, Choi SJ, Choe PG, et al. 2016; First imported case of Zika virus infection into Korea. J Korean Med Sci. 31:1173–7. DOI: 10.3346/jkms.2016.31.7.1173. PMID: 27366020. PMCID: PMC4901014.
Article6. Yoon D, Shin SH, Jang HC, Kim ES, Song EH, Moon SM, et al. 2017; Epidemiology and clinical characteristics of Zika virus infections imported into Korea from March to October 2016. J Korean Med Sci. 32:1440–4. DOI: 10.3346/jkms.2017.32.9.1440. PMID: 28776338. PMCID: PMC5546962.
Article7. Barjas-Castro ML, Angerami RN, Cunha MS, Suzuki A, Nogueira JS, Rocco IM, et al. 2016; Probable transfusion-transmitted Zika virus in Brazil. Transfusion. 56:1684–8. DOI: 10.1111/trf.13681. PMID: 27329551.
Article8. Darrigo LG Jr. 2018; , de Sant'Anna Carvalho AM, Machado CM. Chikungunya, Dengue, and Zika in immunocompromised hosts. Curr Infect Dis Rep. 20:5. DOI: 10.1007/s11908-018-0612-2. PMID: 29551005. PMCID: PMC5857271.
Article9. Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. 2016; Zika virus and birth defects--reviewing the evidence for causality. N Engl J Med. 374:1981–7. DOI: 10.1056/NEJMsr1604338. PMID: 27074377.10. World Health Organization. Zika virus disease: interim case definitions. http://www.who.int/iris/handle/10665/204381. Updated on Feb 2016.11. Bingham AM, Cone M, Mock V, Heberlein-Larson L, Stanek D, Blackmore C, et al. 2016; Comparison of test results for Zika virus RNA in urine, serum, and saliva specimens from persons with travel-associated Zika virus disease - Florida, 2016. MMWR Morb Mortal Wkly Rep. 65:475–8. DOI: 10.15585/mmwr.mm6518e2. PMID: 27171533.
Article12. Lamb LE, Bartolone SN, Kutluay SB, Robledo D, Porras A, Plata M, et al. 2016; Advantage of urine based molecular diagnosis of Zika virus. Int Urol Nephrol. 48:1961–6. DOI: 10.1007/s11255-016-1406-9. PMID: 27567913.
Article13. Musso D, Nhan T, Robin E, Roche C, Bierlaire D, Zisou K, et al. 2014; Potential for Zika virus transmission through blood transfusion demonstrated during an outbreak in French Polynesia, November 2013 to February 2014. Euro Surveill. 19:20761. DOI: 10.2807/1560-7917.ES2014.19.14.20761. PMID: 24739982.
Article14. Balmaseda A, Zambrana JV, Collado D, García N, Saborío S, Elizondo D, et al. 2018; Comparison of four serological methods and two reverse transcription-PCR assays for diagnosis and surveillance of Zika virus infection. J Clin Microbiol. 56:e01785–17. DOI: 10.1128/JCM.01785-17. PMID: 29305550. PMCID: PMC5824050.
Article15. Theel ES, Hata DJ. 2018; Diagnostic testing for Zika virus: a postoutbreak update. J Clin Microbiol. 56:e01972–17. DOI: 10.1128/JCM.01972-17. PMID: 29386264. PMCID: PMC5869840.
Article16. Corman VM, Rasche A, Baronti C, Aldabbagh S, Cadar D, Reusken CB, et al. 2016; Assay optimization for molecular detection of Zika virus. Bull World Health Organ. 94:880–92. DOI: 10.2471/BLT.16.175950. PMID: 27994281. PMCID: PMC5153932.
Article17. Rossini G, Gaibani P, Vocale C, Cagarelli R, Landini MP. 2017; Comparison of Zika virus (ZIKV) RNA detection in plasma, whole blood and urine - Case series of travel-associated ZIKV infection imported to Italy, 2016. J Infect. 75:242–5. DOI: 10.1016/j.jinf.2017.05.021. PMID: 28648495.18. Centers for Disease Control and Prevention. Interim guidance for Zika virus testing of urine-United States, 2016. http://dx.doi.org/10.15585/mmwr.mm6518e1. Updated on May 2016. DOI: 10.15585/mmwr.mm6518e1. PMID: 27171368.