Lab Med Online.
2021 Oct;11(4):254-266. 10.47429/lmo.2021.11.4.254.
Evaluation of Matrix Effect in Body Fluid Chemistry on Roche Cobas 8000 c702 System
- Affiliations
-
- 1Department of Laboratory Medicine, Seoul National University Hospital, Seoul, Korea
- 2Department of Laboratory Medicine, Seoul National University College of Medicine, Seoul, Korea
- 3Department of Laboratory Medicine, Seoul National University Bundang Hospital, Seongnam, Korea
- KMID: 2526087
- DOI: http://doi.org/10.47429/lmo.2021.11.4.254
Abstract
- Background
Analysis of body fluids aids in diagnosis and monitoring disease. However, only a few testing platforms and reagents have been validated for a range of body fluids or analytes. In this study, we evaluated a testing system, which has been approved for blood samples, in analyzing body fluid specimens upon matrix mixing.
Methods
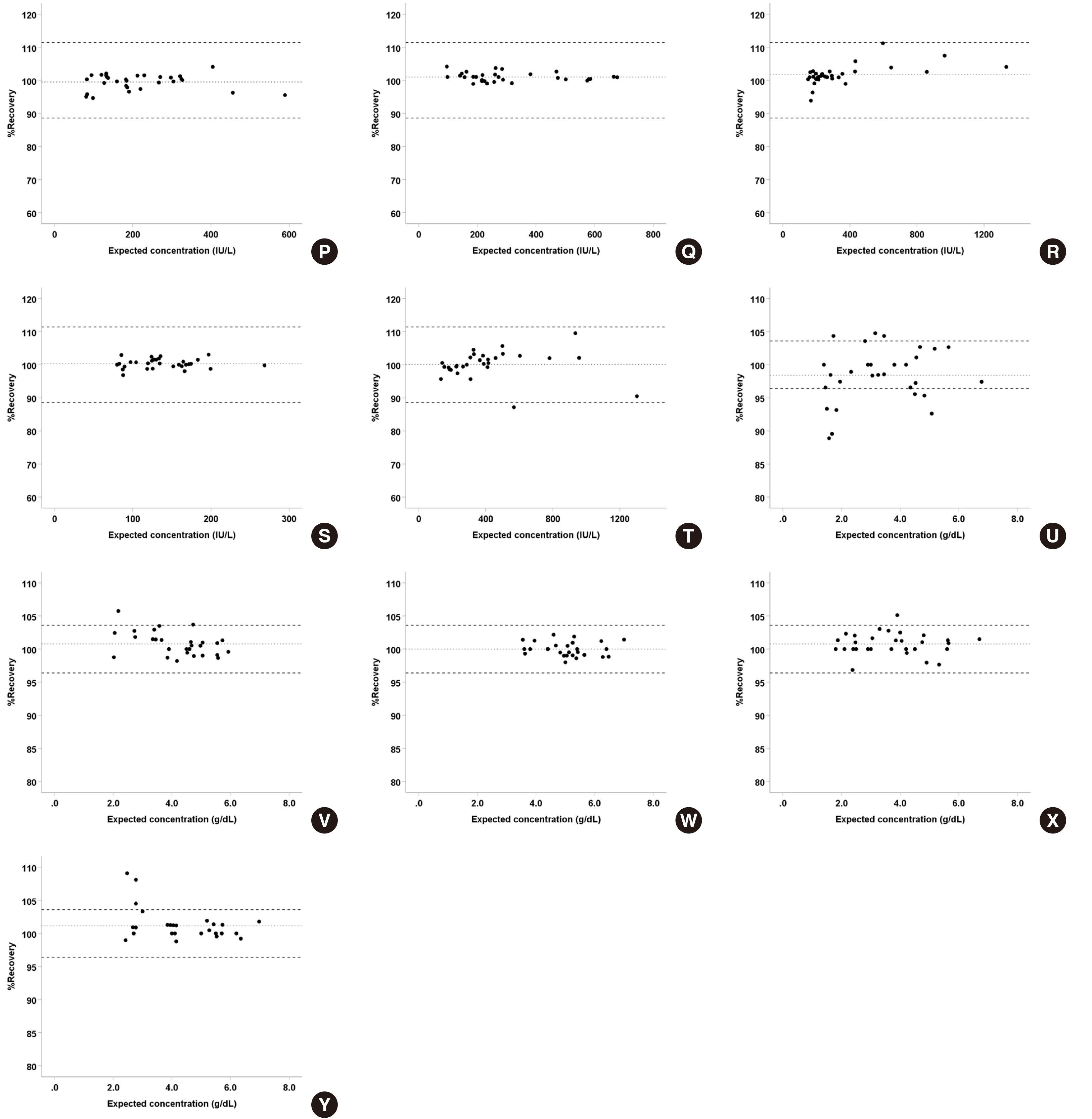
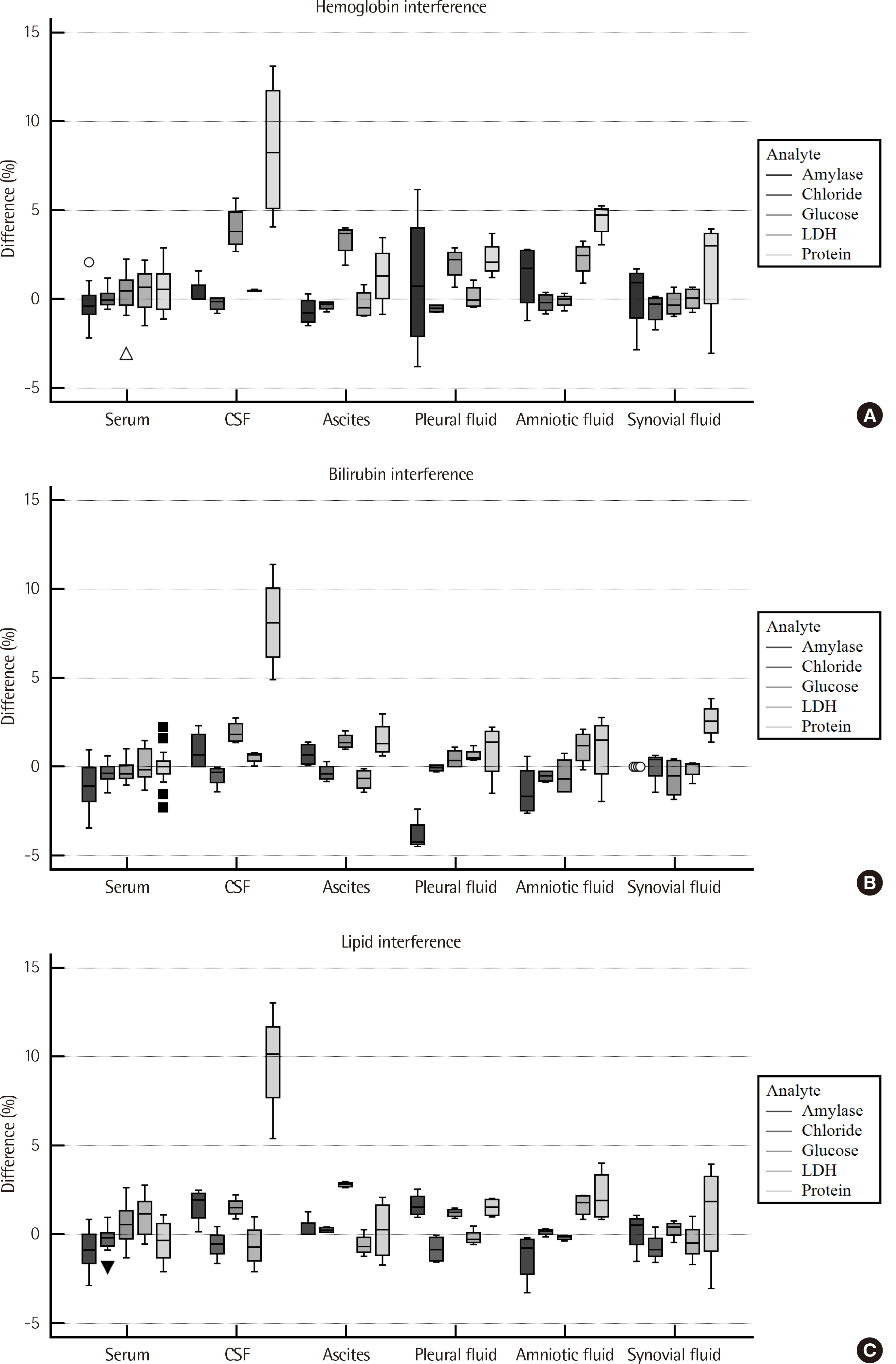
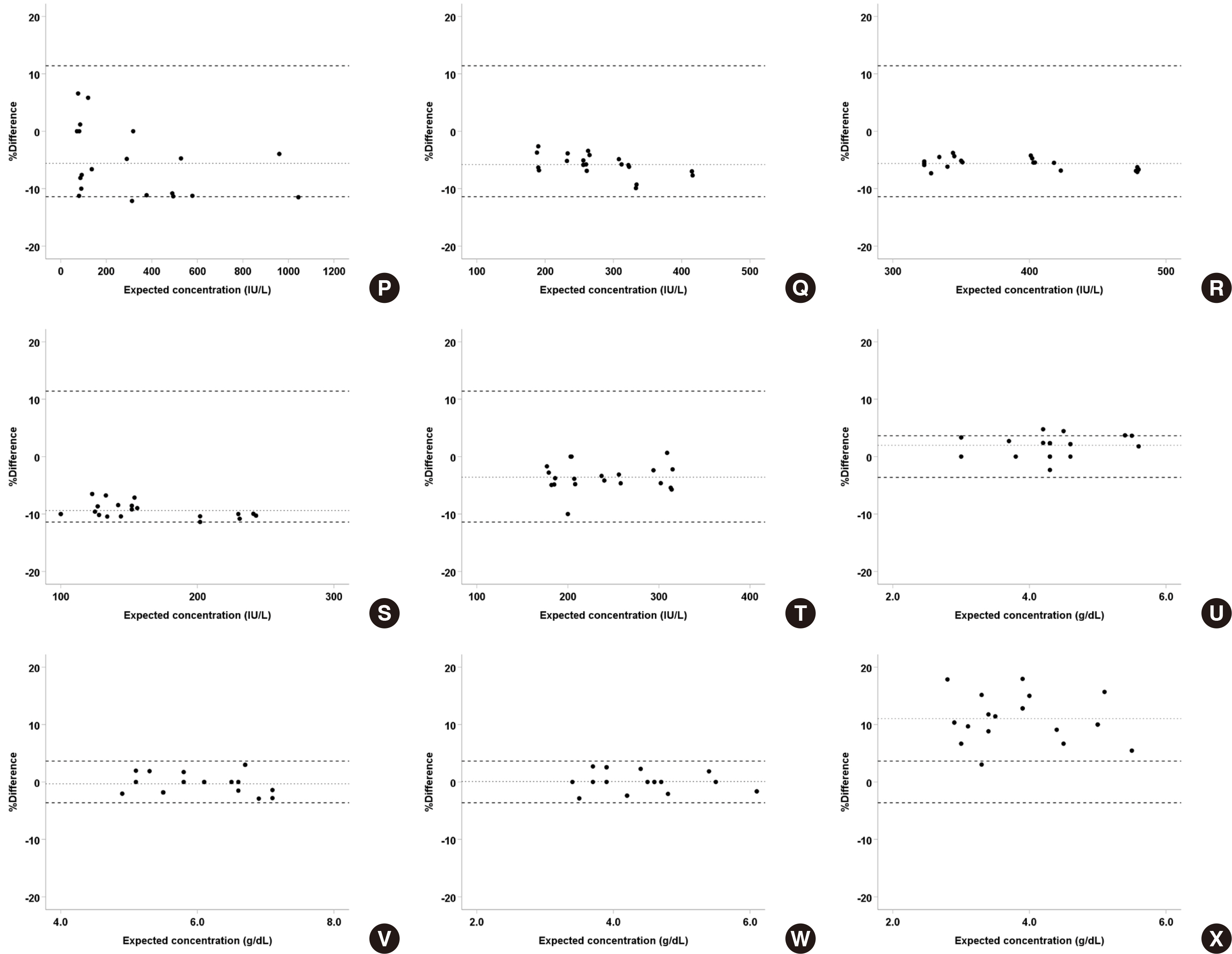
Serum and body fluid samples, including cerebrospinal fluid (CSF), ascites, pleural fluid, amniotic fluid, and synovial fluid, were mixed, then the matrix effect and linearity for major analytes, namely amylase, chloride, glucose, LDH, and protein were evaluated (N = 30 serum-body fluid pairs) on the Cobas 8000 c702. The obtained data was compared with that of open reagents evaluated on the Architect c16000.
Results
For all analyte-body fluid pairs, the mean percent recovery ranged from 98.4% to 101.7%, and this was within the acceptable range for matrix effect. In the linearity test, maximum non-linearity for each analyte-body fluid pair ranged from -5.0% to +4.2%. In interference test, proteins showed positive hemolytic, icteric, and lipemic interference in CSF and hemolytic interference in amniotic fluid. There was no significant interference in the other analyte-body fluid pairs. Results were highly correlated between the Cobas 8000 c702 and the Architect c16000 system.
Conclusions
Our findings revealed that the matrix effect of major analytes in body fluid specimens can be excluded and they also validated the linearity of the analytes in the body fluid specimens. Therefore, reagents specified for blood samples can be readily adopted for the analysis of body fluids.
Figure
Reference
-
1. American Society of Clinical Pathologists. 1999. Handbook of clinical pathology. 2nd ed. American Society of Clinical Pathologists;Chicago, Ill.:2. Wians FH Jr. 2009; Clinical laboratory tests: Which, why, and what do the results mean? Labmedicine. 40:105–13. DOI: 10.1309/LM404L0HHUTWWUDD.
Article3. Block DR, Algeciras-Schimnich A. 2013; Body fluid analysis: clinical utility and applicability of published studies to guide interpretation of today's laboratory testing in serous fluids. Crit Rev Clin Lab Sci. 50:107–24. DOI: 10.3109/10408363.2013.844679. PMID: 24156653.
Article4. Owen WE, Thatcher ML, Crabtree KJ, Greer RW, Strathmann FG, Straseski JA, et al. 2015; Body fluid matrix evaluation on a Roche cobas 8000 system. Clin Biochem. 48:911–4. DOI: 10.1016/j.clinbiochem.2015.05.012. PMID: 26006756.
Article5. Block DR, Ouverson LJ, Wittwer CA, Saenger AK, Baumann NA. 2018; An approach to analytical validation and testing of body fluid assays for the automated clinical laboratory. Clin Biochem. 58:44–52. DOI: 10.1016/j.clinbiochem.2018.05.002. PMID: 29738695.
Article6. Adams A, Straseski JA, Lehman CM, Pearson LN. 2019; Peritoneal and pleural fluid chemistry measurements performed on three chemistry platforms. Lab Med. 50:145–9. DOI: 10.1093/labmed/lmy056. PMID: 30169773.
Article7. Clinical and Laboratory Standards Institute. 2007. Analysis of body fluids in clinical chemistry: Approved guideline. CLSI document C49-A. Clinical and Laboratory Standards Institute;Wayne, PA:8. Swift CP, Gwaikolo C, Ssentamu J, Wachekwa I, Adeiza MA, Adu E, et al. 2019; Body fluid testing at John F. Kennedy Medical Center in Liberia. Am J Clin Pathol. 152:86–90. DOI: 10.1093/ajcp/aqz027. PMID: 31165167.
Article9. Westgard QC. Desirable biological variation database specifications. https://www.westgard.com/biodatabase1. Updated in 2014.10. Clinical and Laboratory Standards Institute. 2003. Evaluation of the linearity of quantitative measurement procedures: a statistical approach; Approved guideline. CLSI document EP06-A. Clinical and Laboratory Standards Institute;Wayne, PA:11. CAP. All common checklist: COM.40620 Body fluid analysis. https://elss.cap.org/elss/ShowProperty?nodePath=/UCMCON/Contribution%20Folders/DctmContent/education/OnlineCourseContent/2017/LAP-TLTM/checklists/cl-com.pdf. Updated on Aug, 2017.12. Lippi G, Plebani M. 2017; Opportunities and drawbacks of nonstandard body fluid analysis. Clin Chem Lab Med. 55:907–9. DOI: 10.1515/cclm-2016-0862. PMID: 27754963.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of Serum Creatinine Measurements among Roche Modular D, Cobas 8000 c702, and Beckman Coulter AU5800, by Jaffe and Enzymatic Methods
- Performance Evaluation of the Roche-Hitachi cobas 8000 c702 Chemistry Autoanalyzer
- Performance Evaluation of the JEOL BioMajesty JCA-BM6010/C Automated Clinical Chemistry Analyzer
- Evaluation of Analytical Performance of the Cobas 8000 Analyzer Series Module e602
- Performance Evaluation of Beckman Coulter AU5822 Automated Clinical Chemistry Analyzer