Cancer Res Treat.
2022 Jan;54(1):10-19. 10.4143/crt.2020.1200.
Validation of the Korean Version of the Patient-Reported Outcomes Measurement Information System 29 Profile V2.1 among Cancer Survivors
- Affiliations
-
- 1Department of Clinical Research Design & Evaluation, SAIHST, Sungkyunkwan University, Seoul, Korea
- 2Center for Clinical Epidemiology, Samsung Medical Center, Seoul, Korea
- 3Department of Digital Health, SAIHST, Sungkyunkwan University, Seoul, Korea
- 4Department of Medical Device Management and Research, SAIHST, Sungkyunkwan University, Seoul, Korea
- 5Clinical Research Institute, Samsung Medical Center, Seoul, Korea
- 6School of Pharmacy, Sungkyunkwan University, Suwon, Korea
- 7Center for Research Resource Standardization, Seoul, Korea
- 8Division of Hematology/Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 9Department of Radiation Oncology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2524583
- DOI: http://doi.org/10.4143/crt.2020.1200
Abstract
- Purpose
The purpose of the study was to validate the Korean version of Patient-Reported Outcomes Measurement Information System 29 Profile v2.1 (K-PROMIS-29 V2.1) among cancer survivors.
Materials and Methods
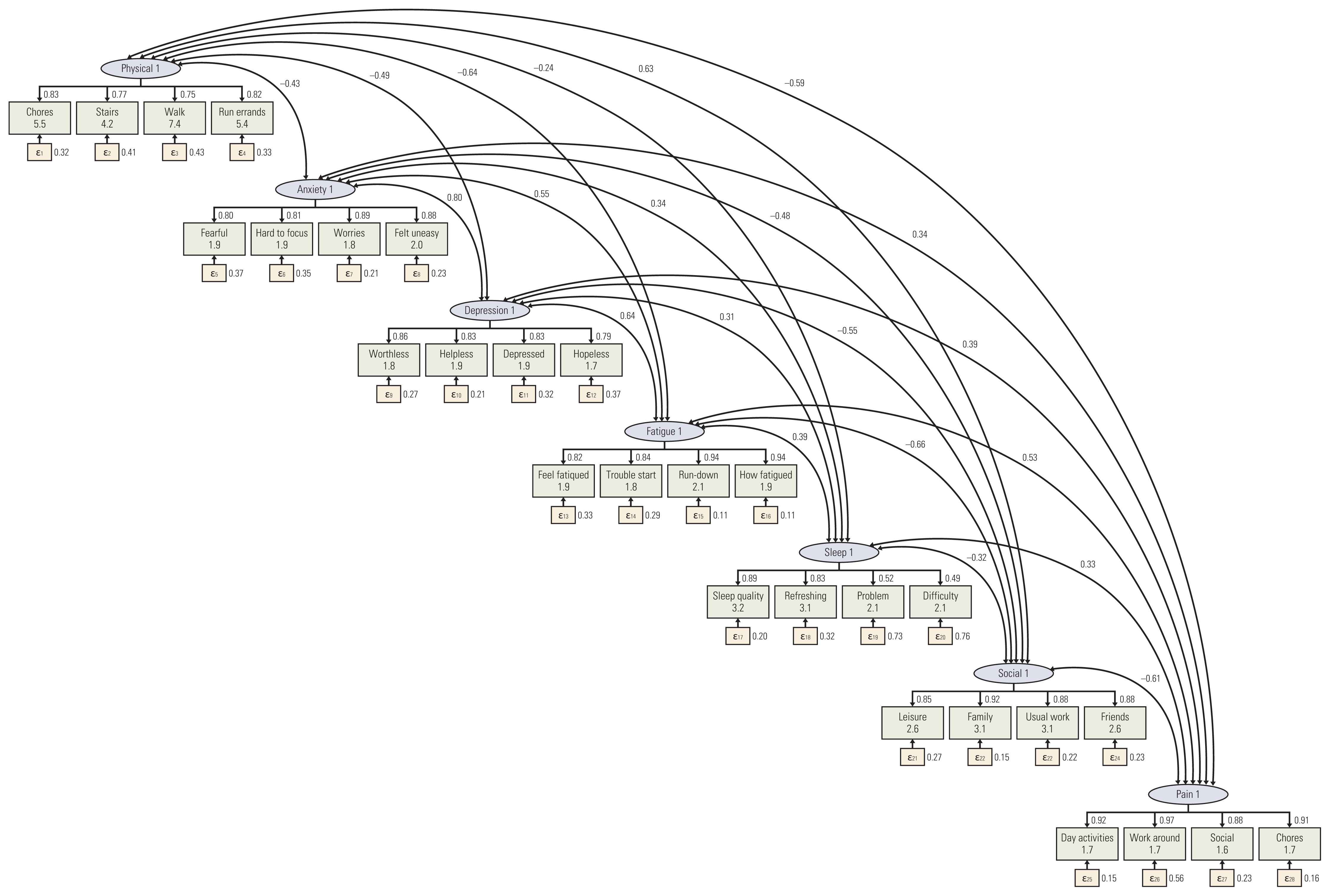
Participants were recruited from outpatient clinics of the Comprehensive Cancer Center at the Samsung Medical Center in Seoul, South Korea, from September to October 2018. Participants completed a survey questionnaire that included the K-PROMIS-29 V2.1 and the European Organisation for Research and Treatment of Cancer Quality of Life Core Questionnaire (EORTC QLQ-C30). Principal component analysis and confirmatory factor analysis (CFA) and Pearson’s correlations were used to evaluate the reliability and validity of the K-PROMIS-29 V2.1.
Results
The mean age of the study participants was 54.4 years, the mean time since diagnosis was 1.2 (±2.4) years, and 349 (87.3%) completed the entire questionnaire. The Cronbach’s alpha coefficients of the seven domains in the K-PROMIS-29 V2.1 ranged from 0.81 to 0.96, indicating satisfactory internal consistency. In the CFA, the goodness-of-fit indices for the K-PROMIS-29 V2.1 were high (comparative fit index, 0.91 and standardized root-mean-squared residual, 0.06). High to moderate correlations were found between comparable subscales of the K-PROMIS-29 V2.1 and subscales of the EORTC QLQ-C30 (r=0.52-0.73).
Conclusion
The K-PROMIS-29 V2.1 is a reliable and valid measure for assessing the health-related quality of life domains in a cancer population, thus supporting their use in studies and oncology trials.
Keyword
Figure
Reference
-
References
1. Kim JY, Lee KE, Kim K, Lee MA, Yoon WS, Han DS, et al. Choosing wisely: the Korean perspective and launch of the ‘right decision in cancer care’ initiative. Cancer Res Treat. 2020; 52:655–60.
Article2. European Medicines Agency. Appendix 2 to the guideline on the evaluation of anticancer medicinal products in man: the use of patient–reported outcome (PRO) measures in oncology studies. Amsterdam: European Medicines Agency;2016.3. Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993; 85:365–76.
Article4. Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993; 11:570–9.
Article5. Sprangers MA, Cull A, Bjordal K, Groenvold M, Aaronson NK. The European Organization for Research and Treatment of Cancer: approach to quality of life assessment: guidelines for developing questionnaire modules. EORTC Study Group on Quality of Life. Qual Life Res. 1993; 2:287–95.6. Jensen RE, Potosky AL, Moinpour CM, Lobo T, Cella D, Hahn EA, et al. United States population-based estimates of patient-reported outcomes measurement information system symptom and functional status reference values for individuals with cancer. J Clin Oncol. 2017; 35:1913–20.
Article7. Brenner H. Long-term survival rates of cancer patients achieved by the end of the 20th century: a period analysis. Lancet. 2002; 360:1131–5.
Article8. Grande GE, Farquhar MC, Barclay SI, Todd CJ. Quality of life measures (EORTC QLQ-C30 and SF-36) as predictors of survival in palliative colorectal and lung cancer patients. Palliat Support Care. 2009; 7:289–97.
Article9. Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992; 30:473–83.10. Brooks R. EuroQol: the current state of play. Health Policy. 1996; 37:53–72.
Article11. Wells GA, Russell AS, Haraoui B, Bissonnette R, Ware CF. Validity of quality of life measurement tools: from generic to disease-specific. J Rheumatol Suppl. 2011; 88:2–6.12. Efthymiadou O, Mossman J, Kanavos P. Health related quality of life aspects not captured by EQ-5D-5L: results from an international survey of patients. Health Policy. 2019; 123:159–65.
Article13. Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol. 2010; 63:1179–94.
Article14. Jensen RE, Potosky AL, Reeve BB, Hahn E, Cella D, Fries J, et al. Validation of the PROMIS physical function measures in a diverse US population-based cohort of cancer patients. Qual Life Res. 2015; 24:2333–44.
Article15. Quach CW, Langer MM, Chen RC, Thissen D, Usinger DS, Emerson MA, et al. Reliability and validity of PROMIS measures administered by telephone interview in a longitudinal localized prostate cancer study. Qual Life Res. 2016; 25:2811–23.
Article16. Fayers PM, Aaronson NK, Bjordal K, Groenvold M, Curran D, Bottomley A. The EORTC QLQ-C30 scoring manual. 3rd ed. Brussels: European Organization for Research and Treatment of Cancer;2001.17. Yun YH, Park YS, Lee ES, Bang SM, Heo DS, Park SY, et al. Validation of the Korean version of the EORTC QLQ-C30. Qual Life Res. 2004; 13:863–8.
Article18. Ursachi G, Horodnic IA, Zait A. How reliable are measurement scales? External factors with indirect influence on reliability estimators. Procedia Econ Financ. 2015; 20:679–86.
Article19. Hu LT, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct Equ Modeling. 1999; 6:1–55.
Article20. Mokkink LB, Terwee CB, Patrick DL, Alonso J, Stratford PW, Knol DL, et al. The COSMIN checklist for assessing the methodological quality of studies on measurement properties of health status measurement instruments: an international Delphi study. Qual Life Res. 2010; 19:539–49.
Article21. Hinkle DE, Wiersma W, Jurs SG. Applied statistics for the behavioral sciences. Boston, MA: Houghton Mifflin;2003.22. van Kooten JA, Terwee CB, Kaspers GJ, van Litsenburg RR. Content validity of the patient-reported outcomes measurement information system sleep disturbance and sleep related impairment item banks in adolescents. Health Qual Life Outcomes. 2016; 14:92.
Article23. Terwee CB, Bot SD, de Boer MR, van der Windt DA, Knol DL, Dekker J, et al. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol. 2007; 60:34–42.
Article24. Dobson KS. The relationship between anxiety and depression. Clin Psychol Rev. 1985; 5:307–24.
Article25. Krueger RF, Finger MS. Using item response theory to understand comorbidity among anxiety and unipolar mood disorders. Psychol Assess. 2001; 13:140–51.
Article26. Libman E, Fichten C, Creti L, Conrod K, Tran DL, Grad R, et al. Refreshing sleep and sleep continuity determine perceived sleep quality. Sleep Disord. 2016; 2016:7170610.
Article27. Hartman JD, Craig BM. Comparing and transforming PROMIS utility values to the EQ-5D. Qual Life Res. 2018; 27:725–33.
Article28. Morrisroe K, Stevens W, Huq M, Sahhar J, Ngian GS, Zochling J, et al. Validity of the workers productivity and activity impairment questionnaire: specific health problem (WPAI: SHP) in patients with systemic sclerosis. Clin Exp Rheumatol. 2017; 35(Suppl 106):130–7.29. Hinchcliff ME, Beaumont JL, Carns MA, Podlusky S, Thavarajah K, Varga J, et al. Longitudinal evaluation of PROMIS-29 and FACIT-dyspnea short forms in systemic sclerosis. J Rheumatol. 2015; 42:64–72.
Article30. Forrest CB, Bevans KB, Tucker C, Riley AW, Ravens-Sieberer U, Gardner W, et al. Commentary: the patient-reported outcome measurement information system (PROMIS(R)) for children and youth: application to pediatric psychology. J Pediatr Psychol. 2012; 37:614–21.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Validation of the Korean Version of the Patient-Reported Outcomes Information System® Emotional Distress Measures
- Patient-Reported Outcomes Measurement Information System: Translation and Linguistic Validation of Six Profile Domains for Korean Adults
- A Literature Review on Unmet Needs of High-Prevalence Cancer Survivors: Focus on Breast Cancer, Thyroid Cancer, Colorectal Cancer, and Lung Cancer
- Prostate Imaging Reporting and Data System (PI-RADS) v 2.1: Overview and Critical Points
- Psychometric Validation of the Korean Version of the Cancer Survivors’ Unmet Needs (CaSUN) Scale among Korean Non–Small Cell Lung Cancer Survivors