Diabetes Metab J.
2021 Nov;45(6):933-947. 10.4093/dmj.2020.0223.
Carnitine Orotate Complex Ameliorates Insulin Resistance and Hepatic Steatosis Through Carnitine Acetyltransferase Pathway
- Affiliations
-
- 1Division of Endocrinology & Metabolism, Samsung Biomedical Research Institute, Seoul, Korea
- 2Division of Endocrinology & Metabolism, Department of Internal Medicine, Uijeongbu Eulji Medical Center, Eulji University School of Medicine, Uijeongbu, Korea
- KMID: 2522732
- DOI: http://doi.org/10.4093/dmj.2020.0223
Abstract
- Background
Carnitine orotate complex (Godex) has been shown to decrease glycated hemoglobin levels and improve steatosis in patients with type 2 diabetes mellitus with non-alcoholic fatty liver disease. However, the mechanisms of Godex in glucose metabolism remain unclear.
Methods
Male C57BL/6J mice were divided into four groups: normal-fat diet, high-fat diet, a high-fat diet supplemented with intraperitoneal injection of (500 mg or 2,000 mg/kg/day) Godex for 8 weeks. Computed tomography, indirect calorimetry, and histological analyses including electron microscopy of the liver were performed, and biochemical profiles and oral glucose tolerance test and insulin tolerance test were undertaken. Expressions of genes in the lipid and glucose metabolism, activities of oxidative phosphorylation enzymes, carnitine acetyltransferase, pyruvate dehydrogenase, and acetyl-coenzyme A (CoA)/CoA ratio were evaluated.
Results
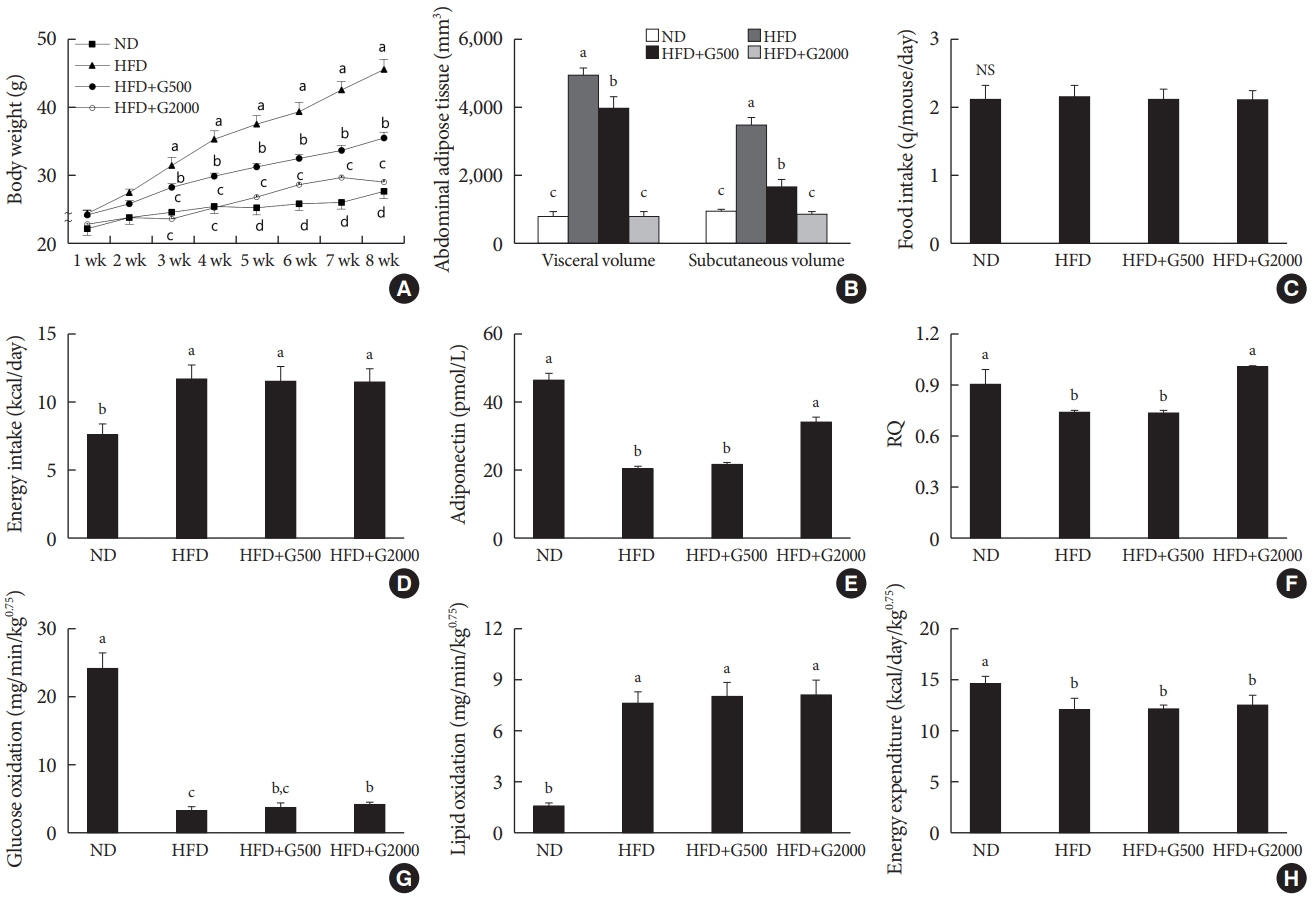
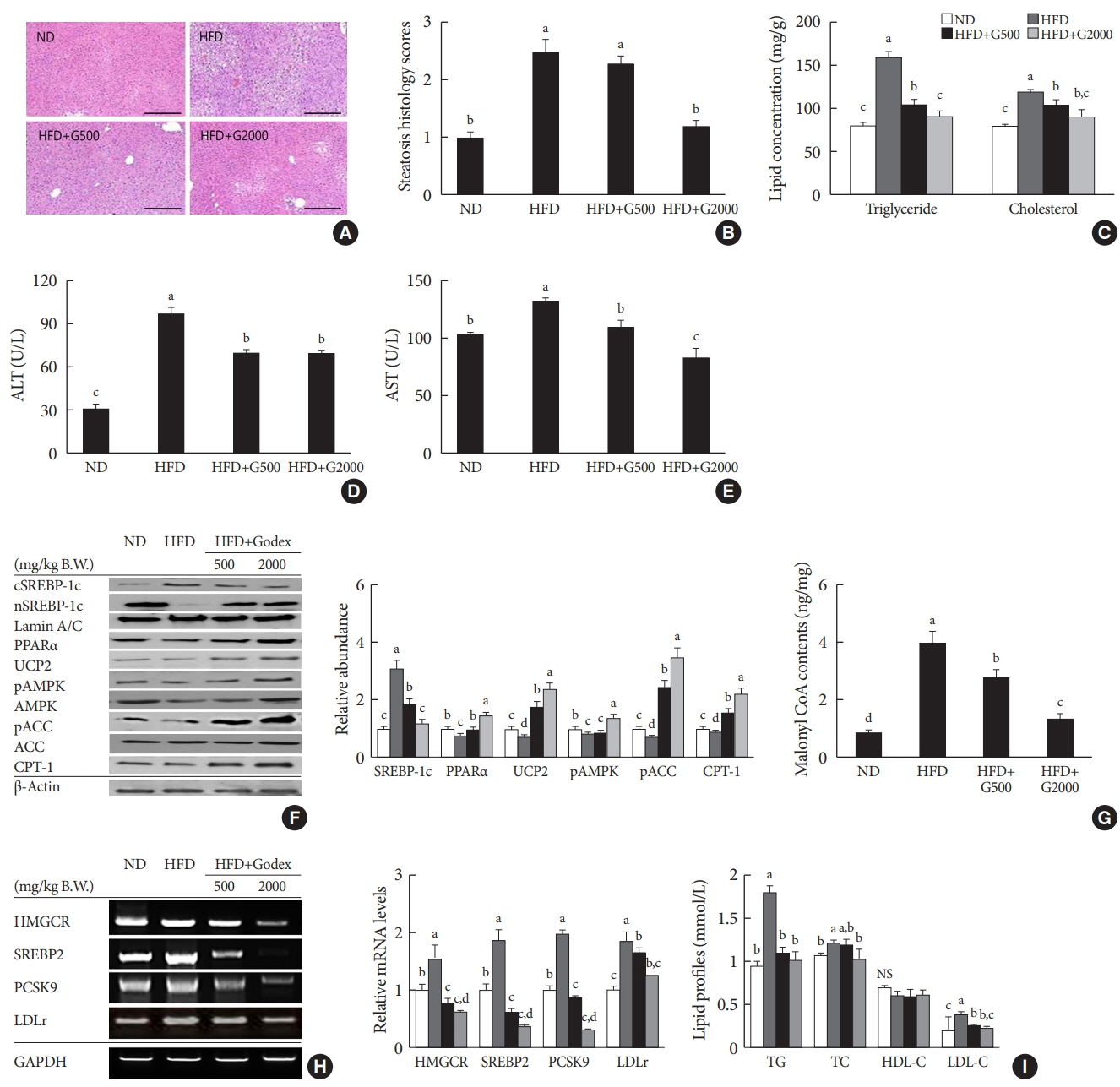
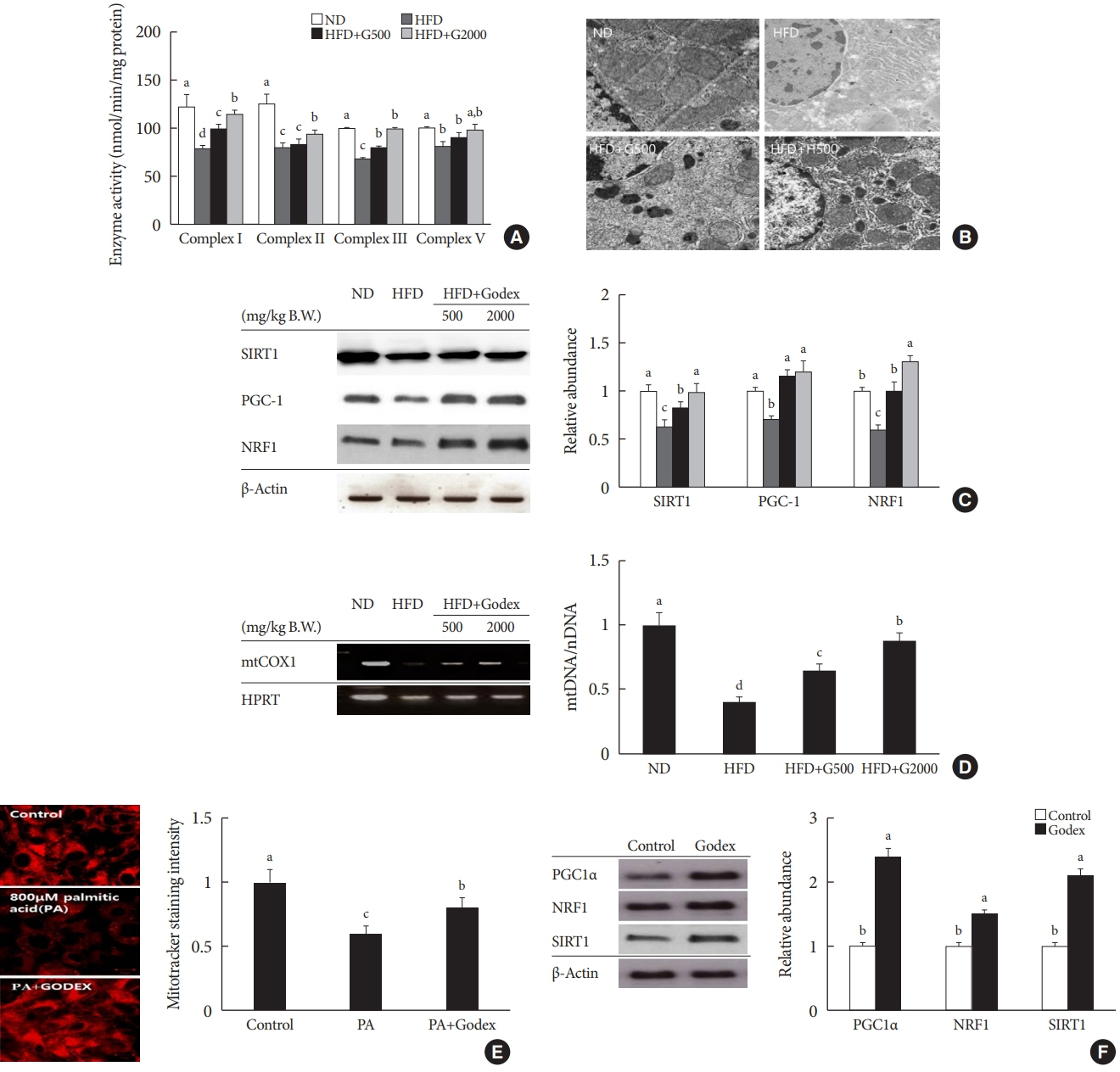
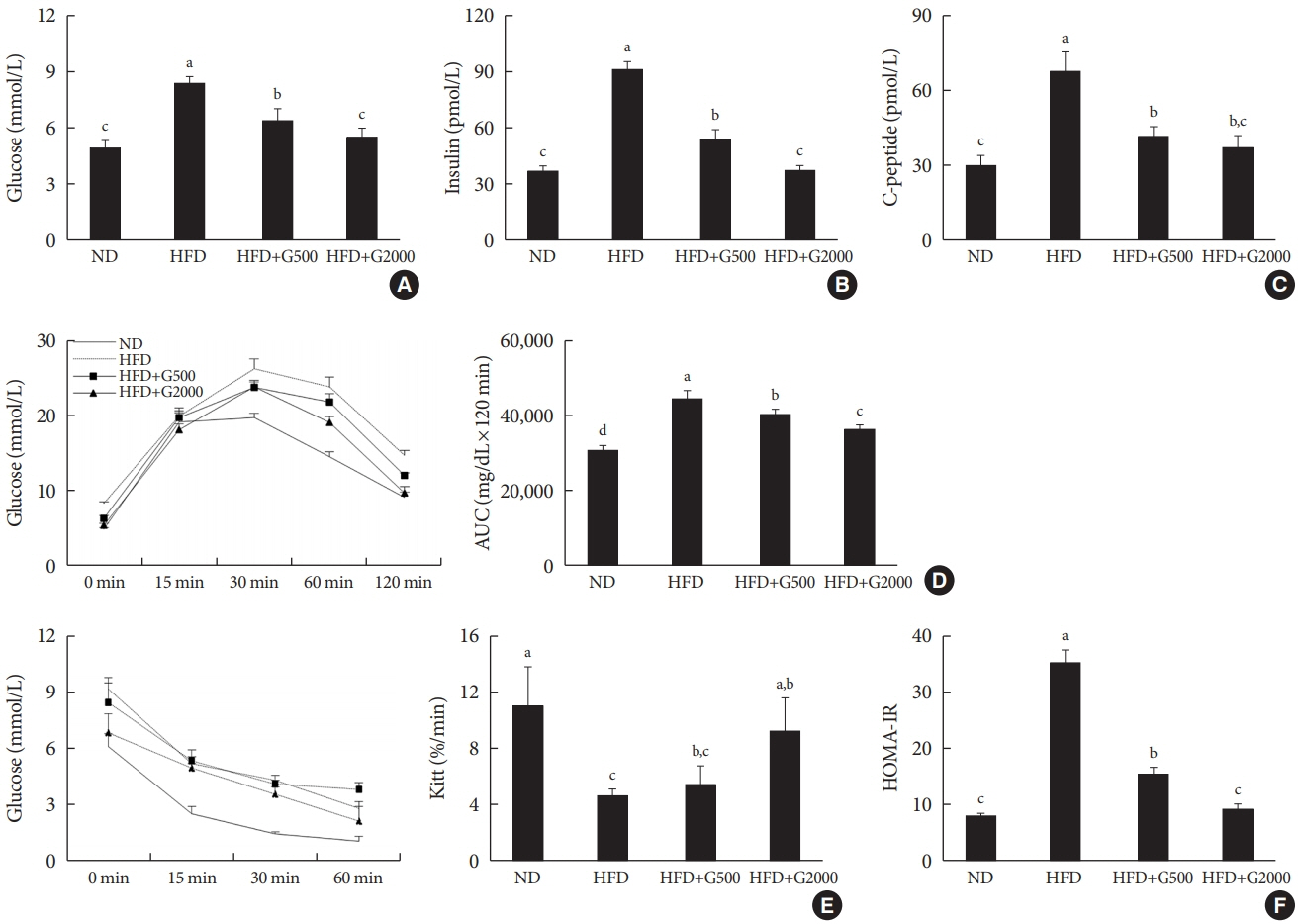
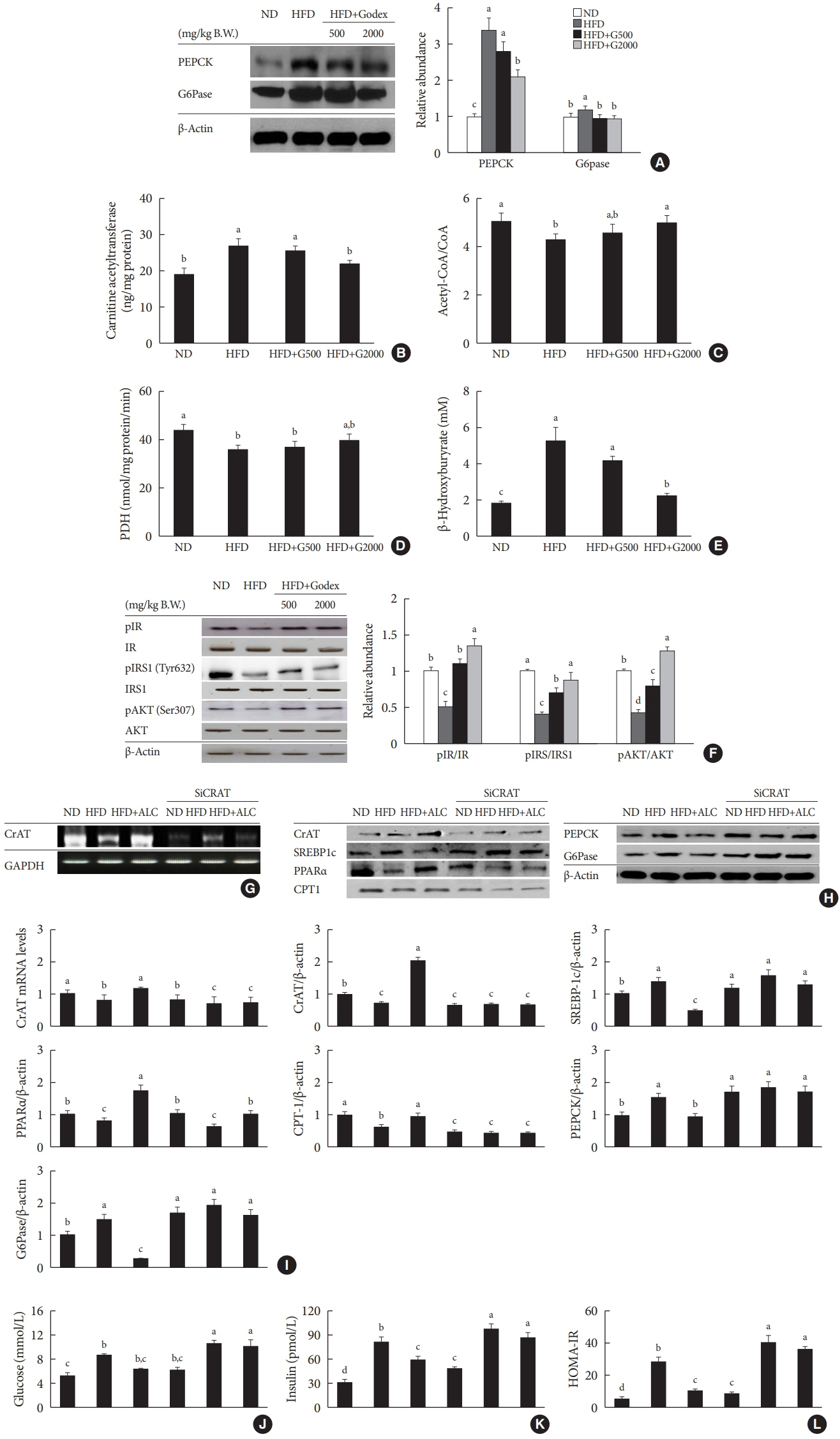
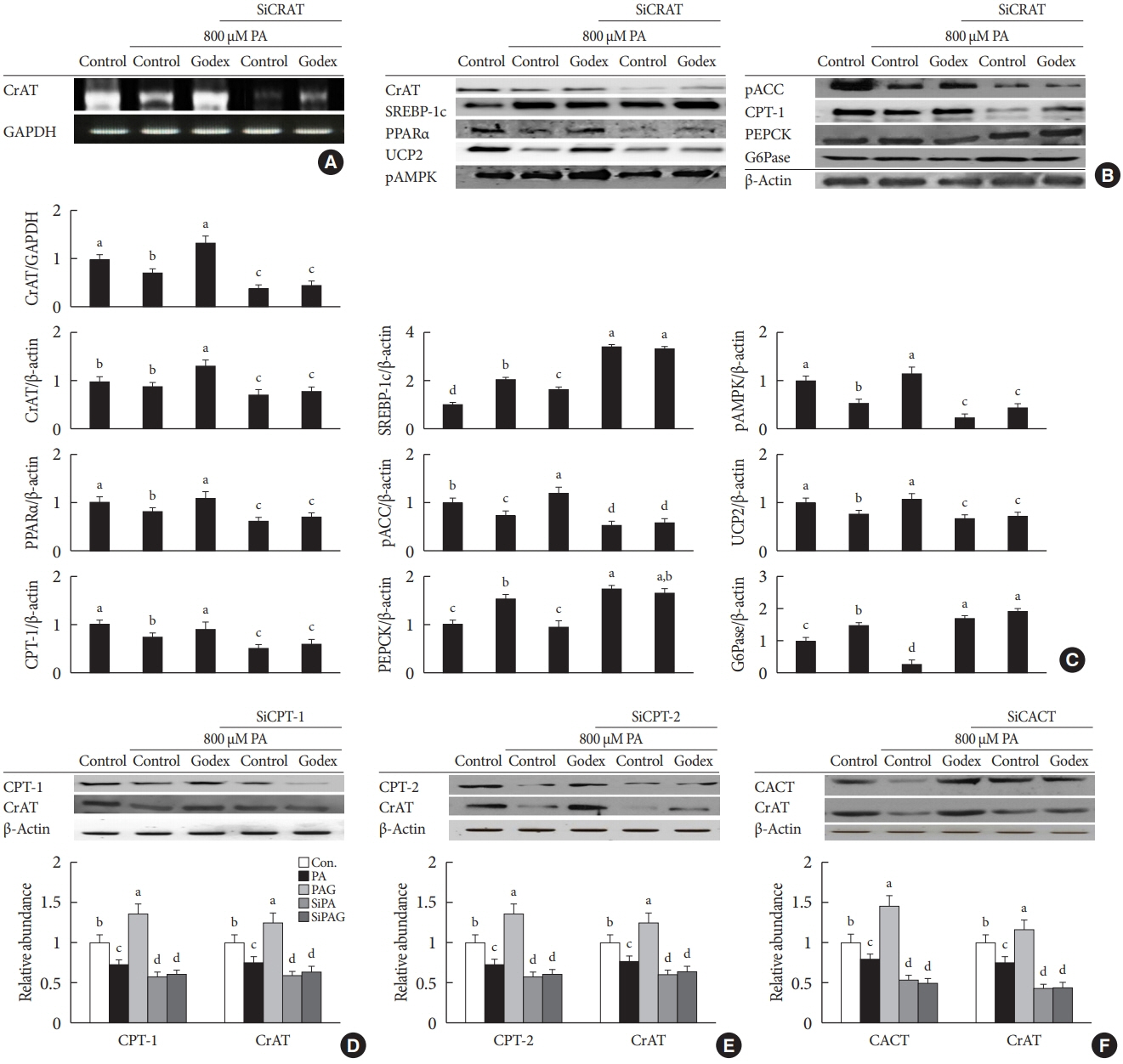
Godex improved insulin sensitivity and significantly decreased fasting plasma glucose, homeostatic model assessment for insulin resistance, steatosis, and gluconeogenesis, with a marked increase in fatty acid oxidation as well as better use of glucose in high-fat diet-fed mice. It preserved mitochondrial function and ultrastructure, restored oxidative phosphorylation enzyme activities, decreased acetyl-CoA/CoA ratio, and increased carnitine acetyltransferase content and pyruvate dehydrogenase activity. Carnitine acetyltransferase knockdown partially reversed the effects of Godex in liver and in vitro.
Conclusion
Godex improved insulin resistance and steatosis by regulating carnitine acetyltransferase in liver in high-fat diet-fed mice.
Figure
Cited by 1 articles
-
The Role of Carnitine Orotate Complex in Fatty Liver
Hyon-Seung Yi
Diabetes Metab J. 2021;45(6):866-867. doi: 10.4093/dmj.2021.0272.
Reference
-
1. Sears B, Perry M. The role of fatty acids in insulin resistance. Lipids Health Dis. 2015; 14:121.
Article2. Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, et al. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001; 120:1183–92.
Article3. Fillmore N, Mori J, Lopaschuk GD. Mitochondrial fatty acid oxidation alterations in heart failure, ischaemic heart disease and diabetic cardiomyopathy. Br J Pharmacol. 2014; 171:2080–90.
Article4. Guo W, Li D, You Y, Li W, Hu B, Zhang S, et al. Cystathionine γ-lyase deficiency aggravates obesity-related insulin resistance-via FoxO1-dependent hepatic gluconeogenesis. FASEB J. 2019; 33:4212–24.5. Park MS, Kang JS, Chon CY, Paik SW, Rim KS, Kwak MJ, et al. Oral Godex capsule for chronic liver disease: a double-blind, randomized, multicenter controlled trial. J Korean Soc Clin Pharmacol Ther. 2001; 9:151–62.6. Jun DW, Kim BI, Cho YK, Kim HJ, Kwon YO, Park SY, et al. Efficacy and safety of entecavir plus carnitine complex (GODEX(R)) compared to entecavir monotherapy in patient with ALT elevated chronic hepatitis B: randomized, multicenter open-label trials: the GOAL study. Clin Mol Hepatol. 2013; 19:165–72.7. Sin JS, Jung EY, Lee MH, Kang JK. Therapeutic effect of the Godex on the liver cirrhosis induced by CCl4 and ethanol in the rat. J Appl Pharmacol. 2002; 10:200–7.8. Hong ES, Kim EK, Kang SM, Khang AR, Choi SH, Park KS, et al. Effect of carnitine-orotate complex on glucose metabolism and fatty liver: a double-blind, placebo-controlled study. J Gastroenterol Hepatol. 2014; 29:1449–57.
Article9. Bae JC, Lee WY, Yoon KH, Park JY, Son HS, Han KA, et al. Improvement of nonalcoholic fatty liver disease with carnitine-orotate complex in type 2 diabetes (CORONA): a randomized controlled trial. Diabetes Care. 2015; 38:1245–52.
Article10. Muoio DM, Noland RC, Kovalik JP, Seiler SE, Davies MN, DeBalsi KL, et al. Muscle-specific deletion of carnitine acetyltransferase compromises glucose tolerance and metabolic flexibility. Cell Metab. 2012; 15:764–77.
Article11. Seiler SE, Martin OJ, Noland RC, Slentz DH, DeBalsi KL, Ilkayeva OR, et al. Obesity and lipid stress inhibit carnitine acetyltransferase activity. J Lipid Res. 2014; 55:635–44.
Article12. Ringseis R, Keller J, Eder K. Role of carnitine in the regulation of glucose homeostasis and insulin sensitivity: evidence from in vivo and in vitro studies with carnitine supplementation and carnitine deficiency. Eur J Nutr. 2012; 51:1–18.
Article13. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972; 18:499–502.
Article14. Attane C, Foussal C, Le Gonidec S, Benani A, Daviaud D, Wanecq E, et al. Apelin treatment increases complete fatty acid oxidation, mitochondrial oxidative capacity, and biogenesis in muscle of insulin-resistant mice. Diabetes. 2012; 61:310–20.
Article15. Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005; 41:1313–21.
Article16. Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957; 226:497–509.
Article17. Kim HS, Noh JH, Hong SH, Hwang YC, Yang TY, Lee MS, et al. Rosiglitazone stimulates the release and synthesis of insulin by enhancing GLUT-2, glucokinase and BETA2/NeuroD expression. Biochem Biophys Res Commun. 2008; 367:623–9.
Article18. Stojkovic M, Machado SA, Stojkovic P, Zakhartchenko V, Hutzler P, Goncalves PB, et al. Mitochondrial distribution and adenosine triphosphate content of bovine oocytes before and after in vitro maturation: correlation with morphological criteria and developmental capacity after in vitro fertilization and culture. Biol Reprod. 2001; 64:904–9.19. Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000; 106:473–81.
Article20. Parra M, Stahl S, Hellmann H. Vitamin B6 and its role in cell metabolism and physiology. Cells. 2018; 7:84.
Article21. Liu Z, Li P, Zhao ZH, Zhang Y, Ma ZM, Wang SX. Vitamin B6 prevents endothelial dysfunction, insulin resistance, and hepatic lipid accumulation in Apoe (-/-) mice fed with high-fat diet. J Diabetes Res. 2016; 2016:1748065.22. Tsuchida A, Yamauchi T, Takekawa S, Hada Y, Ito Y, Maki T, et al. Peroxisome proliferator-activated receptor (PPAR) alpha activation increases adiponectin receptors and reduces obesity-related inflammation in adipose tissue: comparison of activation of PPARalpha, PPARgamma, and their combination. Diabetes. 2005; 54:3358–70.23. Kim SS, Oh HY, Kim HR, Yang JS, Kim DS, Sheen YY, et al. Effect of biphenyl dimethyl dicarboxylate on cytochrome P450 1A1 and 2B1 and CCl4-induced hepatotoxicity in rat liver. Yakhak Hoeji. 1999; 43:827–33.24. Renjith RS, Rajamohan T. Protective and curative effects of Cocos nucifera inflorescence on alloxan-induced pancreatic cytotoxicity in rats. Indian J Pharmacol. 2012; 44:555–9.
Article25. Jocken JW, Langin D, Smit E, Saris WH, Valle C, Hul GB, et al. Adipose triglyceride lipase and hormone-sensitive lipase protein expression is decreased in the obese insulin-resistant state. J Clin Endocrinol Metab. 2007; 92:2292–9.
Article26. Couturier A, Ringseis R, Mooren FC, Kruger K, Most E, Eder K. Carnitine supplementation to obese Zucker rats prevents obesity-induced type II to type I muscle fiber transition and favors an oxidative phenotype of skeletal muscle. Nutr Metab (Lond). 2013; 10:48.
Article27. Bruls YM, de Ligt M, Lindeboom L, Phielix E, Havekes B, Schaart G, et al. Carnitine supplementation improves metabolic flexibility and skeletal muscle acetylcarnitine formation in volunteers with impaired glucose tolerance: a randomised controlled trial. EBio Medicine. 2019; 49:318–30.
Article28. De Gaetano A, Mingrone G, Castagneto M, Calvani M. Carnitine increases glucose disposal in humans. J Am Coll Nutr. 1999; 18:289–95.29. Mingrone G, Greco AV, Capristo E, Benedetti G, Giancaterini A, De Gaetano A, et al. L-carnitine improves glucose disposal in type 2 diabetic patients. J Am Coll Nutr. 1999; 18:77–82.
Article30. Lopaschuk GD. Fatty acid oxidation and its relation with insulin resistance and associated disorders. Ann Nutr Metab. 2016; 68 Suppl 3:15–20.
Article31. Montgomery MK, Brown SH, Mitchell TW, Coster AC, Cooney GJ, Turner N. Association of muscle lipidomic profile with high-fat diet-induced insulin resistance across five mouse strains. Sci Rep. 2017; 7:13914.
Article32. Li Y, Xu S, Mihaylova MM, Zheng B, Hou X, Jiang B, et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011; 13:376–88.
Article33. Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002; 109:1125–31.
Article34. Lagace TA, Curtis DE, Garuti R, McNutt MC, Park SW, Prather HB, et al. Secreted PCSK9 decreases the number of LDL receptors in hepatocytes and in livers of parabiotic mice. J Clin Invest. 2006; 116:2995–3005.35. Zhang DW, Lagace TA, Garuti R, Zhao Z, McDonald M, Horton JD, et al. Binding of proprotein convertase subtilisin/kexin type 9 to epidermal growth factor-like repeat A of low density lipoprotein receptor decreases receptor recycling and increases degradation. J Biol Chem. 2007; 282:18602–12.
Article36. Rong S, Cortes VA, Rashid S, Anderson NN, McDonald JG, Liang G, et al. Expression of SREBP-1c requires SREBP-2-mediated generation of a sterol ligand for LXR in livers of mice. Elife. 2017; 6:e25015.
Article37. Altamimi TR, Thomas PD, Darwesh AM, Fillmore N, Mahmoud MU, Zhang L, et al. Cytosolic carnitine acetyltransferaseas a source of cytosolic acetyl-CoA: a possible mechanism for regulation of cardiac energy metabolism. Biochem J. 2018; 475:959–76.38. Sunny NE, Satapati S, Fu X, He T, Mehdibeigi R, Spring-Robinson C, et al. Progressive adaptation of hepatic ketogenesis in mice fed a high-fat diet. Am J Physiol Endocrinol Metab. 2010; 298:E1226–35.
Article39. Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008; 7:45–56.
Article40. Garcia-Ruiz I, Solis-Munoz P, Fernandez-Moreira D, Grau M, Colina F, Munoz-Yague T, et al. High-fat diet decreases activity of the oxidative phosphorylation complexes and causes nonalcoholic steato hepatitis in mice. Dis Model Mech. 2014; 7:1287–96.41. McCarty M, DiNicolantonio JJ, O’Keefe JH. The ability of carnitine to act as a type 1 histone deacetylase inhibitor may explain the favorable impact of carnitine supplementation on mitochondrial biogenesis in the elderly. Med Res Arch. 2020; 8:1–22.
Article42. Fernandez-Marcos PJ, Auwerx J. Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis. Am J Clin Nutr. 2011; 93:884S–90S.
Article43. Fulco M, Cen Y, Zhao P, Hoffman EP, McBurney MW, Sauve AA, et al. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev Cell. 2008; 14:661–73.
Article44. Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009; 458:1056–60.
Article45. Vega RB, Huss JM, Kelly DP. The coactivator PGC-1 cooperates with peroxisome proliferator-activated receptor alpha in transcriptional control of nuclear genes encoding mitochondrial fatty acid oxidation enzymes. Mol Cell Biol. 2000; 20:1868–76.46. Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005; 434:113–8.47. Purushotham A, Schug TT, Xu Q, Surapureddi S, Guo X, Li X. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab. 2009; 9:327–38.
Article48. Fuller SJ, Randle PJ. Reversible phosphorylation of pyruvate dehydrogenasein rat skeletal-muscle mitochondria: effects of starvation and diabetes. Biochem J. 1984; 219:635–46.49. Uziel G, Garavaglia B, Di Donato S. Carnitine stimulation of pyruvate dehydrogenase complex (PDHC) in isolated human skeletal muscle mitochondria. Muscle Nerve. 1988; 11:720–4.
Article50. Goldberg EL, Dixit VD. Carnitine acetyltransferase (CRAT) expression in macrophages is dispensable for nutrient stress sensing and inflammation. Mol Metab. 2017; 6:219–25.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ceramides: Nutrient Signals that Drive Hepatosteatosis
- L-carnitine in maintenance hemodialysis clinical, lipid and biochemical effects
- The Role of Carnitine Orotate Complex in Fatty Liver
- Effect of L-carnitine on ischemic myocardium of Langendorff's isolated rat heart
- Serum Fatty Acid and Carnitine Levels in Obese Children with Fatty Livers