Ann Clin Neurophysiol.
2021 Oct;23(2):92-98. 10.14253/acn.2021.23.2.92.
Introduction of brain computer interface to neurologists
- Affiliations
-
- 1Department of Neurology, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, Changwon, Korea
- 2Department of Electronics Engineering, Chosun University College of Engineering, Gwangju, Korea
- 3Department of Neurology, Centum Hospital, Jinju, Korea
- 4Department of Neurosurgery, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, Changwon, Korea
- 5Department of Neurology, Gyeongsang National University Changwon Hospital, Gyeongsang National University College of Medicine, Changwon, Korea
- 6Department of Neurology and Institute of Health Science, Gyeongsang National University Hospital, Gyeongsang National University College of Medicine, Jinju, Korea
- KMID: 2521886
- DOI: http://doi.org/10.14253/acn.2021.23.2.92
Abstract
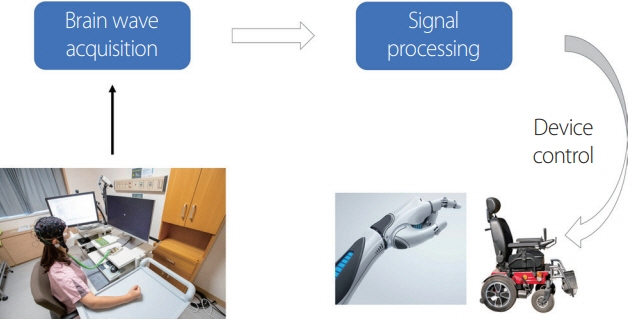
- A brain-computer interface (BCI) is a technology that acquires and analyzes electrical signals from the brain to control external devices. BCI technologies can generally be used to control a computer cursor, limb orthosis, or word processing. This technology can also be used as a neurological rehabilitation tool for people with poor motor control. We reviewed historical attempts and methods toward predicting arm movements using brain waves. In addition, representative studies of minimally invasive and noninvasive BCI were summarized.
Figure
Reference
-
1. Vidal JJ. Toward direct brain-computer communication. Annu Rev Biophys Bioeng. 1973; 2:157–180.
Article2. Nicolelis M. Beyond boundaries: The new neuroscience of connecting brains with machines---and how it will change our lives. 1st ed. New York: Times Books;2011. p. 1–353.3. Rastogi A, Vargas-Irwin CE, Willett FR, Abreu J, Crowder DC, Murphy BA, et al. Neural representation of observed, imagined, and attempted grasping force in motor cortex of individuals with chronic tetraplegia. Sci Rep. 2020; 10:1429.
Article4. Evarts EV. Relation of pyramidal tract activity to force exerted during voluntary movement. J Neurophysiol. 1968; 31:14–27.
Article5. Humphrey DR, Schmidt EM, Thompson WD. Predicting measures of motor performance from multiple cortical spike trains. Science. 1970; 170:758–762.
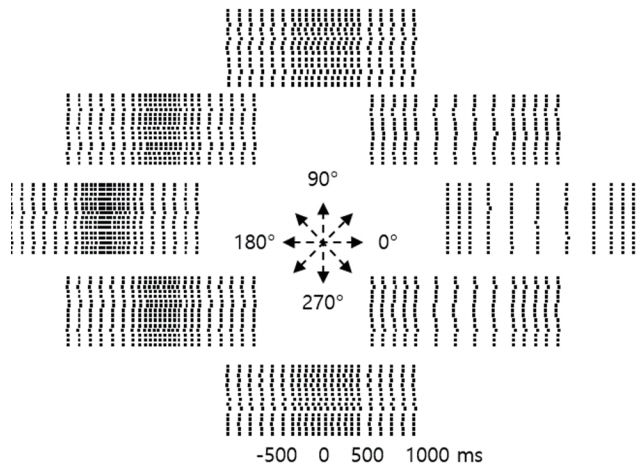
Article6. Georgopoulos AP, Kalaska JF, Caminiti R, Massey JT. On the relations between the direction of two-dimensional arm movements and cell discharge in primate motor cortex. J Neurosci. 1982; 2:1527–1537.
Article7. Georgopoulos AP, Schwartz AB, Kettner RE. Neuronal population coding of movement direction. Science. 1986; 233:1416–1419.
Article8. Georgopoulos AP, Kettner RE, Schwartz AB. Primate motor cortex and free arm movements to visual targets in three-dimensional space. II. Coding of the direction of movement by a neuronal population. J Neurosci. 1988; 8:2928–2937.9. Wessberg J, Stambaugh CR, Kralik JD, Beck PD, Laubach M, Chapin JK, et al. Real-time prediction of hand trajectory by ensembles of cortical neurons in primates. Nature. 2000; 408:361–365.
Article10. Flint RD, Lindberg EW, Jordan LR, Miller LE, Slutzky MW. Accurate decoding of reaching movements from field potentials in the absence of spikes. J Neural Eng. 2012; 9:046006.
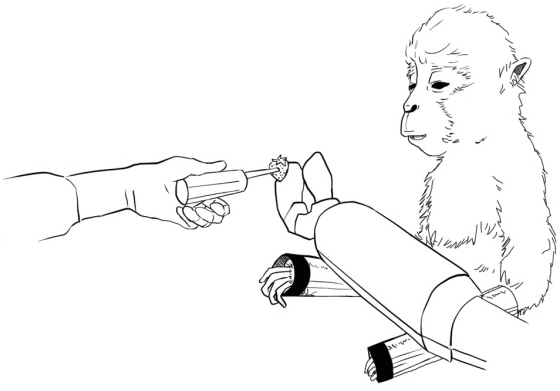
Article11. Velliste M, Perel S, Spalding MC, Whitford AS, Schwartz AB. Cortical control of a prosthetic arm for self-feeding. Nature. 2008; 453:1098–1101.
Article12. Hochberg LR, Bacher D, Jarosiewicz B, Masse NY, Simeral JD, Vogel J, et al. Reach and grasp by people with tetraplegia using a neurally controlled robotic arm. Nature. 2012; 485:372–375.
Article13. Collinger JL, Wodlinger B, Downey JE, Wang W, Tyler-Kabara EC, Weber DJ, et al. High-performance neuroprosthetic control by an individual with tetraplegia. Lancet. 2013; 381:557–564.
Article14. Bouton CE, Shaikhouni A, Annetta NV, Bockbrader MA, Friedenberg DA, Nielson DM, et al. Restoring cortical control of functional movement in a human with quadriplegia. Nature. 2016; 533:247–250.
Article15. Flesher SN, Downey JE, Weiss JM, Hughes CL, Herrera AJ, Tyler-Kabara EC, et al. A brain-computer interface that evokes tactile sensations improves robotic arm control. Science. 2021; 372:831–836.
Article16. Polikov VS, Tresco PA, Reichert WM. Response of brain tissue to chronically implanted neural electrodes. J Neurosci Methods. 2005; 148:1–18.
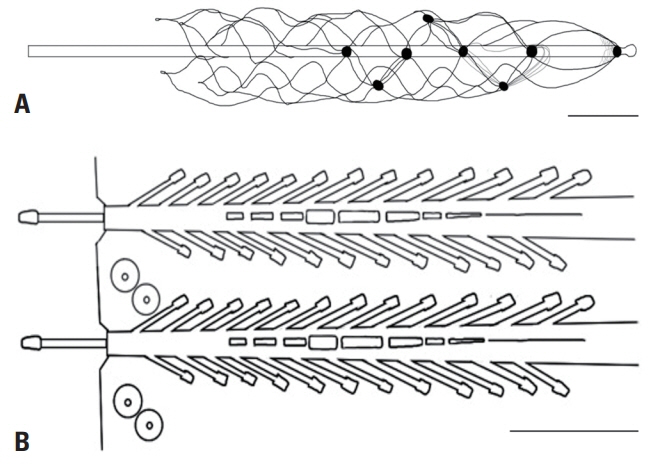
Article17. Oxley TJ, Opie NL, John SE, Rind GS, Ronayne SM, Wheeler TL, et al. Minimally invasive endovascular stent-electrode array for high-fidelity, chronic recordings of cortical neural activity. Nat Biotechnol. 2016; 34:320–327.18. Oxley TJ, Yoo PE, Rind GS, Ronayne SM, Lee CMS, Bird C, et al. Motor neuroprosthesis implanted with neurointerventional surgery improves capacity for activities of daily living tasks in severe paralysis: first in-human experience. J Neurointerv Surg. 2021; 13:102–108.19. Musk E. An integrated brain-machine interface platform with thousands of channels. J Med Internet Res. 2019; 21:e16194.
Article20. Vidal JJ. Real-time detection of brain events in EEG. Proc IEEE. 1977; 65:633–641.
Article21. Wolpaw JR, Birbaumer N, McFarland DJ, Pfurtscheller G, Vaughan TM. Brain-computer interfaces for communication and control. Clin Neurophysiol. 2002; 113:767–791.
Article22. Birbaumer N, Ghanayim N, Hinterberger T, Iversen I, Kotchoubey B, Kübler A, et al. A spelling device for the paralysed. Nature. 1999; 398:297–298.
Article23. Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol. 1999; 110:1842–1857.
Article24. Farwell LA, Donchin E. Talking off the top of your head: toward a mental prosthesis utilizing event-related brain potentials. Electroencephalogr Clin Neurophysiol. 1988; 70:510–523.
Article25. Wang Y, Gao X, Hong B, Jia C, Gao S. Brain-computer interfaces based on visual evoked potentials. IEEE Eng Med Biol Mag. 2008; 27:64–71.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Illiteracy of Brain-Computer Interface
- Brain–computer interface in critical care and rehabilitation
- Basics of Electroencephalography for Neuropsychiatrist
- Can computerized tests be introduced to the Korean Medical Licensing Examination?
- Brain-machine Interface in Robot-assisted Neurorehabilitation for Patients with Stroke and Upper Extremity Weakness – the Therapeutic Turning Point