Cancer Res Treat.
2021 Oct;53(4):962-972. 10.4143/crt.2020.1053.
Role of Interleukin-7 in the Development of and Recovery from Radiation-Induced Lymphopenia: A Post-hoc Analysis of a Prospective Cohort
- Affiliations
-
- 1Department of Radiation Oncology, Yonsei Cancer Center, Yonsei University College of Medicine, Seoul, Korea
- 2Department of Radiation Oncology, Ajou University Medical Center, Suwon, Korea
- KMID: 2521572
- DOI: http://doi.org/10.4143/crt.2020.1053
Abstract
- Purpose
Radiation-induced lymphopenia is associated with worse outcomes in solid tumors. We assessed the impact of interleukin-7 (IL-7), a key cytokine in lymphocyte homeostasis, on radiation-induced lymphopenia.
Materials and Methods
A post-hoc analysis was performed in a prospective cohort of 98 patients with hepatocellular carcinoma who were treated with radiotherapy in 2016-2018. Blood IL-7 levels were assayed before and at the end of radiotherapy. Acute severe lymphopenia (ASL) was defined as a total lymphocyte count of < 200/μL during radiotherapy. Cox and logistic regression analyses were performed to identify predictors of survival and ASL development, respectively.
Results
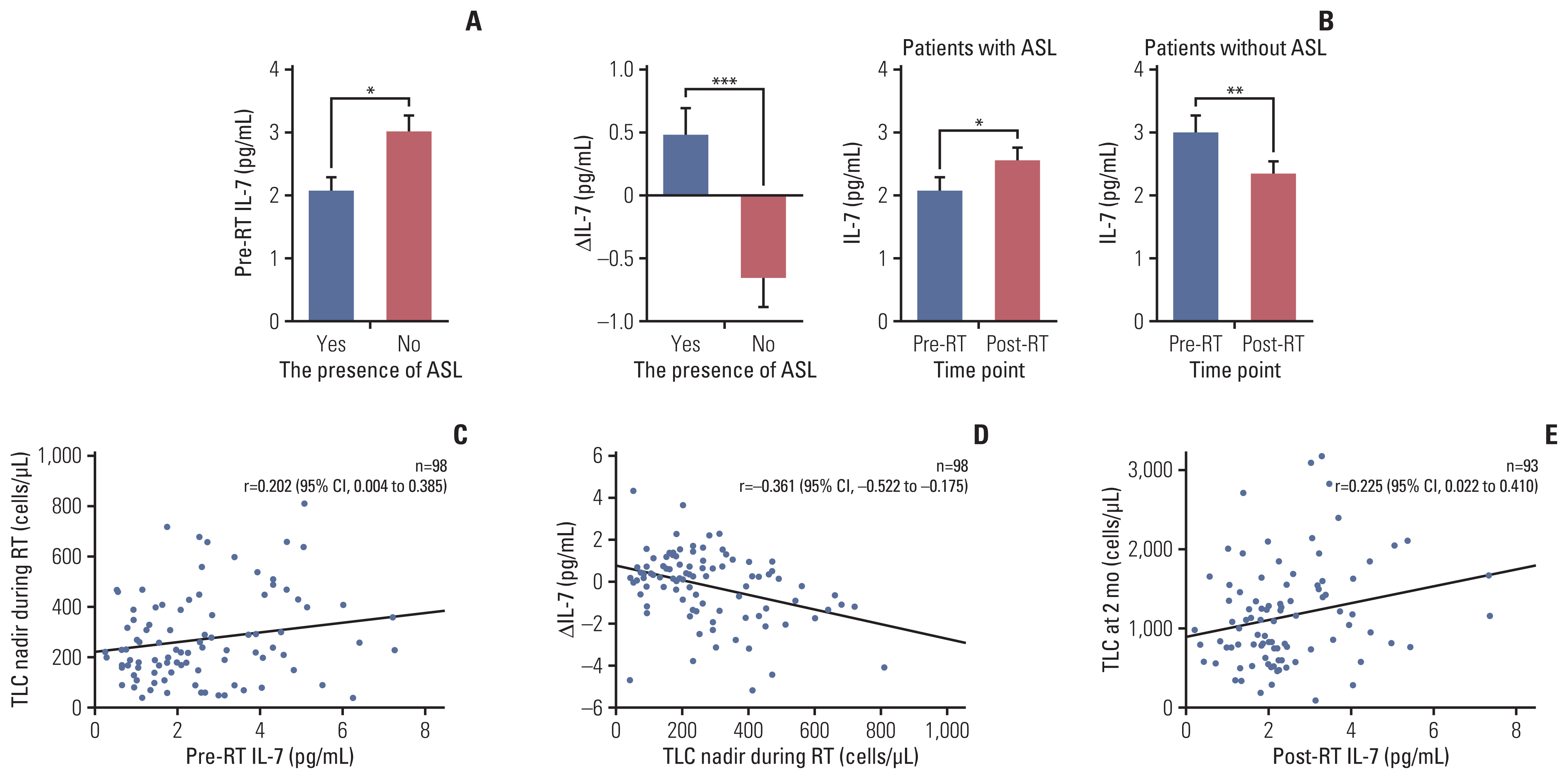
Patients with ASL (n=41) had significantly poorer overall survival than those without (12.0 months vs. 25.3 months, p=0.001). Patients with lymphocyte recovery showed significantly longer overall survival than those without (21.8 months vs. 10.3 months, p=0.042). ASL was an independent predictor of poor survival (hazard ratio, 2.07; p=0.015). Patients with ASL had significantly lower pre-radiotherapy IL-7 levels (2.07 pg/mL vs. 3.01 pg/mL, p=0.010). A high pre-radiotherapy IL-7 level was an independent predictor of a reduced risk of ASL development (hazard ratio, 0.40; p=0.004). IL-7 levels reflected a feedback response to ASL, with a higher ΔIL-7 in patients with ASL and a lower ΔIL-7 in those without ASL (0.48 pg/mL vs. –0.66 pg/mL, p < 0.001). Post-radiotherapy IL-7 levels were significantly positively correlated with the total lymphocyte counts at 2 months.
Conclusion
IL-7 is associated with the development of and recovery from ASL, which may impact survival. To overcome radiation-induced lymphopenia, a novel strategy using IL-7 may be considered.
Keyword
Figure
Reference
-
References
1. Grossman SA, Ellsworth S, Campian J, Wild AT, Herman JM, Laheru D, et al. Survival in patients with severe lymphopenia following treatment with radiation and chemotherapy for newly diagnosed solid tumors. J Natl Compr Canc Netw. 2015; 13:1225–31.
Article2. Byun HK, Kim N, Yoon HI, Kang SG, Kim SH, Cho J, et al. Clinical predictors of radiation-induced lymphopenia in patients receiving chemoradiation for glioblastoma: clinical usefulness of intensity-modulated radiotherapy in the immuno-oncology era. Radiat Oncol. 2019; 14:51.
Article3. Park S, Byun HK, Seong J. Irradiation-related lymphopenia for bone metastasis from hepatocellular carcinoma. Liver Cancer. 2019; 8:468–79.
Article4. Cho Y, Park S, Byun HK, Lee CG, Cho J, Hong MH, et al. Impact of treatment-related lymphopenia on immunotherapy for advanced non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2019; 105:1065–73.
Article5. Byun HK, Kim N, Park S, Seong J. Acute severe lymphopenia by radiotherapy is associated with reduced overall survival in hepatocellular carcinoma. Strahlenther Onkol. 2019; 195:1007–17.
Article6. Liu H, Wang H, Wu J, Wang Y, Zhao L, Li G, et al. Lymphocyte nadir predicts tumor response and survival in locally advanced rectal cancer after neoadjuvant chemoradiotherapy: Immunologic relevance. Radiother Oncol. 2019; 131:52–9.
Article7. Pahl J, Cerwenka A. Tricking the balance: NK cells in anti-cancer immunity. Immunobiology. 2017; 222:11–20.
Article8. Tsukumo SI, Yasutomo K. Regulation of CD8(+) T cells and antitumor immunity by Notch signaling. Front Immunol. 2018; 9:101.
Article9. Gubin MM, Zhang X, Schuster H, Caron E, Ward JP, Noguchi T, et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014; 515:577–81.
Article10. Button LN, DeWolf WC, Newburger PE, Jacobson MS, Kevy SV. The effects of irradiation on blood components. Transfusion. 1981; 21:419–26.
Article11. Nakamura N, Kusunoki Y, Akiyama M. Radiosensitivity of CD4 or CD8 positive human T-lymphocytes by an in vitro colony formation assay. Radiat Res. 1990; 123:224–7.
Article12. Ma A, Koka R, Burkett P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu Rev Immunol. 2006; 24:657–79.
Article13. Rosenberg SA, Sportes C, Ahmadzadeh M, Fry TJ, Ngo LT, Schwarz SL, et al. IL-7 administration to humans leads to expansion of CD8+ and CD4+ cells but a relative decrease of CD4+ T-regulatory cells. J Immunother. 2006; 29:313–9.
Article14. Duane FK, McGale P, Teoh S, Mortimer C, Broggio J, Darby SC, et al. International variation in criteria for internal mammary chain radiotherapy. Clin Oncol (R Coll Radiol). 2019; 31:453–61.
Article15. Ponchel F, Cuthbert RJ, Goeb V. IL-7 and lymphopenia. Clin Chim Acta. 2011; 412:7–16.
Article16. Han KH, Seong J, Kim JK, Ahn SH, Lee DY, Chon CY. Pilot clinical trial of localized concurrent chemoradiation therapy for locally advanced hepatocellular carcinoma with portal vein thrombosis. Cancer. 2008; 113:995–1003.
Article17. Dean RM, Fry T, Mackall C, Steinberg SM, Hakim F, Fowler D, et al. Association of serum interleukin-7 levels with the development of acute graft-versus-host disease. J Clin Oncol. 2008; 26:5735–41.
Article18. Shiraishi Y, Fang P, Xu C, Song J, Krishnan S, Koay EJ, et al. Severe lymphopenia during neoadjuvant chemoradiation for esophageal cancer: a propensity matched analysis of the relative risk of proton versus photon-based radiation therapy. Radiother Oncol. 2018; 128:154–60.
Article19. Huang J, DeWees TA, Badiyan SN, Speirs CK, Mullen DF, Fergus S, et al. Clinical and dosimetric predictors of acute severe lymphopenia during radiation therapy and concurrent temozolomide for high-grade glioma. Int J Radiat Oncol Biol Phys. 2015; 92:1000–7.
Article20. Wild AT, Herman JM, Dholakia AS, Moningi S, Lu Y, Rosati LM, et al. Lymphocyte-sparing effect of stereotactic body radiation therapy in patients with unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys. 2016; 94:571–9.
Article21. Yovino S, Kleinberg L, Grossman SA, Narayanan M, Ford E. The etiology of treatment-related lymphopenia in patients with malignant gliomas: modeling radiation dose to circulating lymphocytes explains clinical observations and suggests methods of modifying the impact of radiation on immune cells. Cancer Invest. 2013; 31:140–4.
Article22. Mazzucchelli R, Durum SK. Interleukin-7 receptor expression: intelligent design. Nat Rev Immunol. 2007; 7:144–54.
Article23. Hakim FT, Gress RE. Reconstitution of the lymphocyte compartment after lymphocyte depletion: a key issue in clinical immunology. Eur J Immunol. 2005; 35:3099–102.
Article24. Napolitano LA, Burt TD, Bacchetti P, Barron Y, French AL, Kovacs A, et al. Increased circulating interleukin-7 levels in HIV-1-infected women. J Acquir Immune Defic Syndr. 2005; 40:581–4.
Article25. Ellsworth S, Balmanoukian A, Kos F, Nirschl CJ, Nirschl TR, Grossman SA, et al. Sustained CD4(+) T cell-driven lymphopenia without a compensatory IL-7/IL-15 response among high-grade glioma patients treated with radiation and temozolomide. Oncoimmunology. 2014; 3:e27357.26. Levy Y, Sereti I, Tambussi G, Routy JP, Lelievre JD, Delfraissy JF, et al. Effects of recombinant human interleukin 7 on T-cell recovery and thymic output in HIV-infected patients receiving antiretroviral therapy: results of a phase I/IIa randomized, placebo-controlled, multicenter study. Clin Infect Dis. 2012; 55:291–300.27. Study of the effect NT-I7 on CD4 counts in patients with high grade gliomas [Internet]. Bethesda, MD: National Library of Medicine;2016. [cited 2020 Jan 2]. Available from: https://clinicaltrials.gov/ct2/show/NCT02659800 .28. Diehl A, Yarchoan M, Hopkins A, Jaffee E, Grossman SA. Relationships between lymphocyte counts and treatment-related toxicities and clinical responses in patients with solid tumors treated with PD-1 checkpoint inhibitors. Oncotarget. 2017; 8:114268–80.
Article29. Demaria S, Golden EB, Formenti SC. Role of local radiation therapy in cancer immunotherapy. JAMA Oncol. 2015; 1:1325–32.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Unraveling the Impact of Sarcopenia-Induced Lymphopenia on Treatment Response and Prognosis in Patients with Stage III Non–Small Cell Lung Cancer: Insights for Optimizing Chemoradiation and Immune Checkpoint Inhibitor
- Lymphopenia following pancreaticoduodenectomy is associated with pancreatic fistula formation
- The Relationship between Radiation-induced Apoptosis and the Expression of Cytokines in the Small Intestine of Rats
- Effect of Cumulative Dexamethasone Dose during Concomitant Chemoradiation on Lymphopenia in Patients with Newly Diagnosed Glioblastoma
- The Relationship between Radiation-Induced Apoptosis and theExpression of Cytokines in the Rat's Liver