Korean J Physiol Pharmacol.
2021 Nov;25(6):565-574. 10.4196/kjpp.2021.25.6.565.
Kir4.1 is coexpressed with stemness markers in activated astrocytes in the injured brain and a Kir4.1 inhibitor BaCl 2 negatively regulates neurosphere formation in culture
- Affiliations
-
- 1Neuroscience Graduate Program, Department of Biomedical Sciences, Ajou University School of Medicine, Suwon 16499, Korea.
- 2Department of Pharmacology, Ajou University School of Medicine, Suwon 16499, Korea.
- 3Chronic Inflammatory Disease Research Center, Ajou University School of Medicine, Suwon 16499, Korea.
- 4Center for Convergence Research of Neurological Disorders, Ajou University School of Medicine, Suwon 16499, Korea.
- 5Department of Brain Science, Ajou University School of Medicine, Suwon 16499, Korea.
- KMID: 2521482
- DOI: http://doi.org/10.4196/kjpp.2021.25.6.565
Abstract
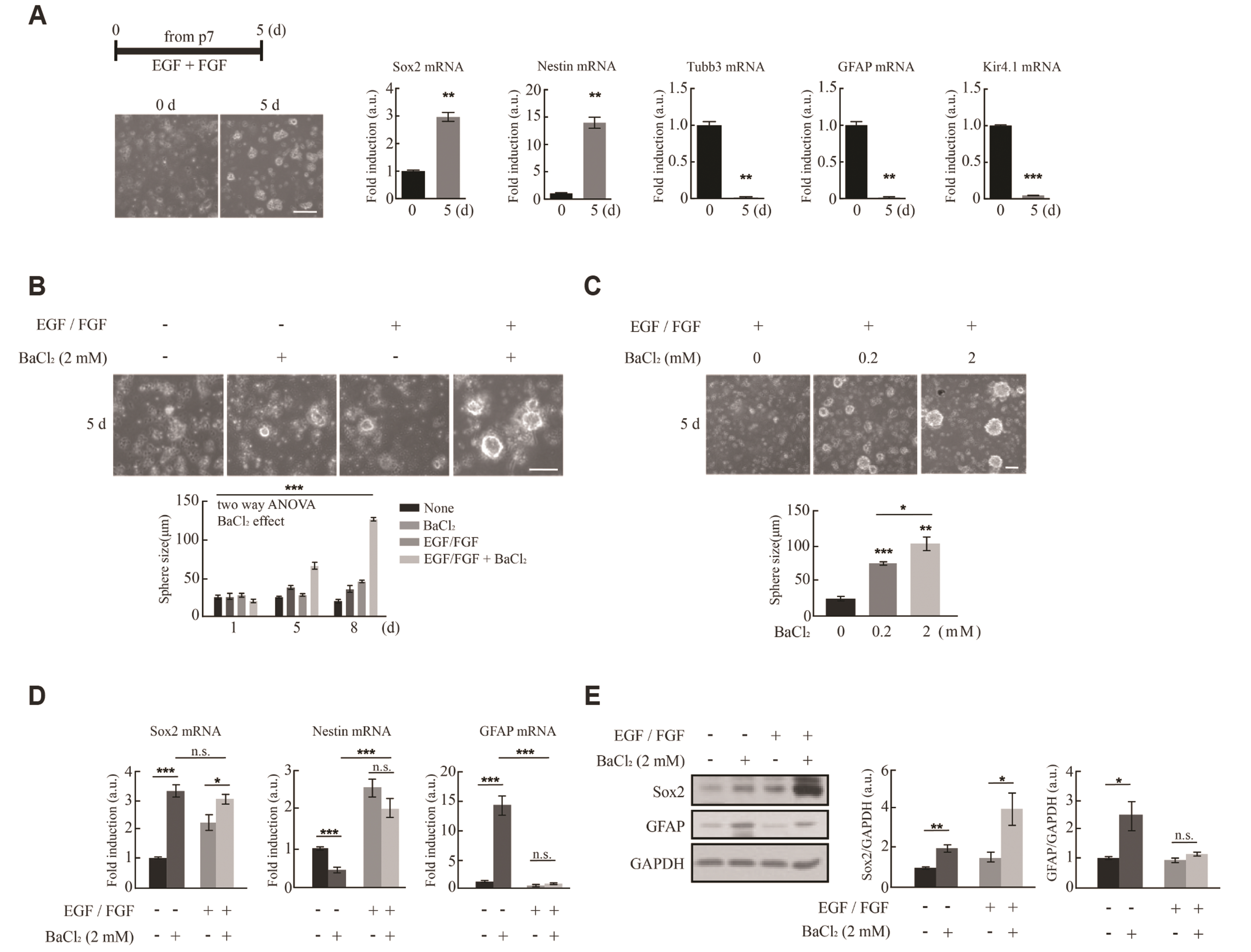
- Astrocytes are activated in response to brain damage. Here, we found that expression of Kir4.1, a major potassium channel in astrocytes, is increased in activated astrocytes in the injured brain together with upregulation of the neural stem cell markers, Sox2 and Nestin. Expression of Kir4.1 was also increased together with that of Nestin and Sox2 in neurospheres formed from dissociated P7 mouse brains. Using the Kir4.1 blocker BaCl2 to determine whether Kir4.1 is involved in acquisition of stemness, we found that inhibition of Kir4.1 activity caused a concentration-dependent increase in sphere size and Sox2 levels, but had little effect on Nestin levels. Moreover, induction of differentiation of cultured neural stem cells by withdrawing epidermal growth factor and fibroblast growth factor from the culture medium caused a sharp initial increase in Kir4.1 expression followed by a decrease, whereas Sox2 and Nestin levels continuously decreased. Inhibition of Kir4.1 had no effect on expression levels of Sox2 or Nestin, or the astrocyte and neuron markers glial fibrillary acidic protein and β-tubulin III, respectively. Taken together, these results indicate that Kir4.1 may control gain of stemness but not differentiation of stem cells.
Keyword
Figure
Reference
-
1. Kofuji P, Ceelen P, Zahs KR, Surbeck LW, Lester HA, Newman EA. 2000; Genetic inactivation of an inwardly rectifying potassium channel (Kir4.1 subunit) in mice: phenotypic impact in retina. J Neurosci. 20:5733–5740. DOI: 10.1523/JNEUROSCI.20-15-05733.2000. PMID: 10908613. PMCID: PMC2410027.
Article2. Poopalasundaram S, Knott C, Shamotienko OG, Foran PG, Dolly JO, Ghiani CA, Gallo V, Wilkin GP. 2000; Glial heterogeneity in expression of the inwardly rectifying K+ channel, Kir4.1, in adult rat CNS. Glia. 30:362–372. DOI: 10.1002/(SICI)1098-1136(200006)30:4<362::AID-GLIA50>3.0.CO;2-4. PMID: 10797616.3. Yakushigawa H, Tokunaga Y, Inanobe A, Kani K, Kurachi Y, Maeda T. 1998; A novel junction-like membrane complex in the optic nerve astrocyte of the Japanese macaque with a possible relation to a potassium ion channel. Anat Rec. 250:465–474. DOI: 10.1002/(SICI)1097-0185(199804)250:4<465::AID-AR10>3.0.CO;2-M. PMID: 9566537.
Article4. Nagelhus EA, Mathiisen TM, Ottersen OP. 2004; Aquaporin-4 in the central nervous system: cellular and subcellular distribution and coexpression with KIR4.1. Neuroscience. 129:905–913. DOI: 10.1016/j.neuroscience.2004.08.053. PMID: 15561407.
Article5. Djukic B, Casper KB, Philpot BD, Chin LS, McCarthy KD. 2007; Conditional knock-out of Kir4.1 leads to glial membrane depolarization, inhibition of potassium and glutamate uptake, and enhanced short-term synaptic potentiation. J Neurosci. 27:11354–11365. DOI: 10.1523/JNEUROSCI.0723-07.2007. PMID: 17942730. PMCID: PMC6673037.
Article6. Cui Y, Yang Y, Ni Z, Dong Y, Cai G, Foncelle A, Ma S, Sang K, Tang S, Li Y, Shen Y, Berry H, Wu S, Hu H. 2018; Astroglial Kir4.1 in the lateral habenula drives neuronal bursts in depression. Nature. 554:323–327. DOI: 10.1038/nature25752. PMID: 29446379.
Article7. Tong X, Ao Y, Faas GC, Nwaobi SE, Xu J, Haustein MD, Anderson MA, Mody I, Olsen ML, Sofroniew MV, Khakh BS. 2014; Astrocyte Kir4.1 ion channel deficits contribute to neuronal dysfunction in Huntington's disease model mice. Nat Neurosci. 17:694–703. DOI: 10.1038/nn.3691. PMID: 24686787. PMCID: PMC4064471.
Article8. ivastava R Sr, Aslam M, Kalluri SR, Schirmer L, Buck D, Tackenberg B, Rothhammer V, Chan A, Gold R, Berthele A, Bennett JL, Korn T, Hemmer B. 2012; Potassium channel KIR4.1 as an immune target in multiple sclerosis. N Engl J Med. 367:115–123. DOI: 10.1056/NEJMoa1110740. PMID: 22784115. PMCID: PMC5131800.
Article9. Urrego D, Tomczak AP, Zahed F, Stühmer W, Pardo LA. 2014; Potassium channels in cell cycle and cell proliferation. Philos Trans R Soc Lond B Biol Sci. 369:20130094. DOI: 10.1098/rstb.2013.0094. PMID: 24493742. PMCID: PMC3917348.
Article10. Yasuda T, Bartlett PF, Adams DJ. 2008; Kir and Kv channels regulate electrical properties and proliferation of adult neural precursor cells. Mol Cell Neurosci. 37:284–297. DOI: 10.1016/j.mcn.2007.10.003. PMID: 18023363.11. Pekny M, Nilsson M. 2005; Astrocyte activation and reactive gliosis. Glia. 50:427–434. DOI: 10.1002/glia.20207. PMID: 15846805.
Article12. Eng LF, Ghirnikar RS. 1994; GFAP and astrogliosis. Brain Pathol. 4:229–237. DOI: 10.1111/j.1750-3639.1994.tb00838.x. PMID: 7952264.
Article13. Kernie SG, Erwin TM, Parada LF. 2001; Brain remodeling due to neuronal and astrocytic proliferation after controlled cortical injury in mice. J Neurosci Res. 66:317–326. DOI: 10.1002/jnr.10013. PMID: 11746349.
Article14. Duan CL, Liu CW, Shen SW, Yu Z, Mo JL, Chen XH, Sun FY. 2015; Striatal astrocytes transdifferentiate into functional mature neurons following ischemic brain injury. Glia. 63:1660–1670. DOI: 10.1002/glia.22837. PMID: 26031629. PMCID: PMC5033006.
Article15. Götz M, Sirko S, Beckers J, Irmler M. 2015; Reactive astrocytes as neural stem or progenitor cells: in vivo lineage, in vitro potential, and genome-wide expression analysis. Glia. 63:1452–1468. DOI: 10.1002/glia.22850. PMID: 25965557. PMCID: PMC5029574.
Article16. Magnusson JP, Göritz C, Tatarishvili J, Dias DO, Smith EM, Lindvall O, Kokaia Z, Frisén J. 2014; A latent neurogenic program in astrocytes regulated by Notch signaling in the mouse. Science. 346:237–241. DOI: 10.1126/science.346.6206.237. PMID: 25301628.
Article17. Sirko S, Behrendt G, Johansson PA, Tripathi P, Costa M, Bek S, Heinrich C, Tiedt S, Colak D, Dichgans M, Fischer IR, Plesnila N, Staufenbiel M, Haass C, Snapyan M, Saghatelyan A, Tsai LH, Fischer A, Grobe K, Dimou L, et al. 2013; Reactive glia in the injured brain acquire stem cell properties in response to sonic hedgehog. [corrected]. Cell Stem Cell. 12:426–439. DOI: 10.1016/j.stem.2013.01.019. PMID: 23561443.18. Shimada IS, LeComte MD, Granger JC, Quinlan NJ, Spees JL. 2012; Self-renewal and differentiation of reactive astrocyte-derived neural stem/progenitor cells isolated from the cortical peri-infarct area after stroke. J Neurosci. 32:7926–7940. DOI: 10.1523/JNEUROSCI.4303-11.2012. PMID: 22674268. PMCID: PMC3398807.
Article19. Buffo A, Rite I, Tripathi P, Lepier A, Colak D, Horn AP, Mori T, Götz M. 2008; Origin and progeny of reactive gliosis: a source of multipotent cells in the injured brain. Proc Natl Acad Sci U S A. 105:3581–3586. DOI: 10.1073/pnas.0709002105. PMID: 18299565. PMCID: PMC2265175.
Article20. Zappone MV, Galli R, Catena R, Meani N, De Biasi S, Mattei E, Tiveron C, Vescovi AL, Lovell-Badge R, Ottolenghi S, Nicolis SK. 2000; Sox2 regulatory sequences direct expression of a (beta)-geo transgene to telencephalic neural stem cells and precursors of the mouse embryo, revealing regionalization of gene expression in CNS stem cells. Development. 127:2367–2382. DOI: 10.1242/dev.127.11.2367. PMID: 10804179.
Article21. Bani-Yaghoub M, Tremblay RG, Lei JX, Zhang D, Zurakowski B, Sandhu JK, Smith B, Ribecco-Lutkiewicz M, Kennedy J, Walker PR, Sikorska M. 2006; Role of Sox2 in the development of the mouse neocortex. Dev Biol. 295:52–66. DOI: 10.1016/j.ydbio.2006.03.007. PMID: 16631155.
Article22. Grazioli S, Pugin J. 2018; Mitochondrial damage-associated molecular patterns: from inflammatory signaling to human diseases. Front Immunol. 9:832. DOI: 10.3389/fimmu.2018.00832. PMID: 29780380. PMCID: PMC5946030.
Article23. Schaefer L. 2014; Complexity of danger: the diverse nature of damage-associated molecular patterns. J Biol Chem. 289:35237–35245. DOI: 10.1074/jbc.R114.619304. PMID: 25391648. PMCID: PMC4271212.
Article24. Le Feuvre RA, Brough D, Touzani O, Rothwell NJ. 2003; Role of P2X7 receptors in ischemic and excitotoxic brain injury in vivo. J Cereb Blood Flow Metab. 23:381–384. DOI: 10.1097/01.WCB.0000048519.34839.97. PMID: 12621313.
Article25. Zheng LM, Zychlinsky A, Liu CC, Ojcius DM, Young JD. 1991; Extracellular ATP as a trigger for apoptosis or programmed cell death. J Cell Biol. 112:279–288. DOI: 10.1083/jcb.112.2.279. PMID: 1988462. PMCID: PMC2288818.
Article26. Yang H, An J, Choi I, Lee K, Park SM, Jou I, Joe EH. 2020; Region-specific astrogliosis: differential vessel formation contributes to different patterns of astrogliosis in the cortex and striatum. Mol Brain. 13:103. DOI: 10.1186/s13041-020-00642-0. PMID: 32698847. PMCID: PMC7374828.
Article27. Jeong HK, Ji KM, Min KJ, Choi I, Choi DJ, Jou I, Joe EH. 2014; Astrogliosis is a possible player in preventing delayed neuronal death. Mol Cells. 37:345–355. DOI: 10.14348/molcells.2014.0046. PMID: 24802057. PMCID: PMC4012084.
Article28. Jeong HK, Ji KM, Kim B, Kim J, Jou I, Joe EH. 2010; Inflammatory responses are not sufficient to cause delayed neuronal death in ATP-induced acute brain injury. PLoS One. 5:e13756. DOI: 10.1371/journal.pone.0013756. PMID: 21060796. PMCID: PMC2966428.
Article29. Suzuki S, Namiki J, Shibata S, Mastuzaki Y, Okano H. 2010; The neural stem/progenitor cell marker nestin is expressed in proliferative endothelial cells, but not in mature vasculature. J Histochem Cytochem. 58:721–730. DOI: 10.1369/jhc.2010.955609. PMID: 20421592. PMCID: PMC2907277.
Article30. Lendahl U, Zimmerman LB, McKay RD. 1990; CNS stem cells express a new class of intermediate filament protein. Cell. 60:585–595. DOI: 10.1016/0092-8674(90)90662-X. PMID: 1689217.
Article31. Olsen ML, Sontheimer H. 2008; Functional implications for Kir4.1 channels in glial biology: from K+ buffering to cell differentiation. J Neurochem. 107:589–601. DOI: 10.1111/j.1471-4159.2008.05615.x. PMID: 18691387. PMCID: PMC2581639.32. Higashimori H, Sontheimer H. 2007; Role of Kir4.1 channels in growth control of glia. Glia. 55:1668–1679. DOI: 10.1002/glia.20574. PMID: 17876807. PMCID: PMC2040118.
Article33. Lan JY, Williams C, Levin M, Black LD 3rd. 2014; Depolarization of cellular resting membrane potential promotes neonatal cardiomyocyte proliferation in vitro. Cell Mol Bioeng. 7:432–445. DOI: 10.1007/s12195-014-0346-7. PMID: 25295125. PMCID: PMC4185190.
Article34. Frederiksen K, McKay RD. 1988; Proliferation and differentiation of rat neuroepithelial precursor cells in vivo. J Neurosci. 8:1144–1151. DOI: 10.1523/JNEUROSCI.08-04-01144.1988. PMID: 3357014. PMCID: PMC6569254.
Article35. Avilion AA, Nicolis SK, Pevny LH, Perez L, Vivian N, Lovell-Badge R. 2003; Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev. 17:126–140. DOI: 10.1101/gad.224503. PMID: 12514105. PMCID: PMC195970.
Article36. Goldshmit Y, Frisca F, Pinto AR, Pébay A, Tang JK, Siegel AL, Kaslin J, Currie PD. 2014; Fgf2 improves functional recovery-decreasing gliosis and increasing radial glia and neural progenitor cells after spinal cord injury. Brain Behav. 4:187–200. DOI: 10.1002/brb3.172. PMID: 24683512. PMCID: PMC3967535.
Article37. Catterall WA. 2011; Voltage-gated calcium channels. Cold Spring Harb Perspect Biol. 3:a003947. DOI: 10.1101/cshperspect.a003947. PMID: 21746798. PMCID: PMC3140680.
Article38. Man JHK, Groenink L, Caiazzo M. 2018; Cell reprogramming approaches in gene- and cell-based therapies for Parkinson's disease. J Control Release. 286:114–124. DOI: 10.1016/j.jconrel.2018.07.017. PMID: 30026082.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Role of Aquaporin-4 in Cerebral Edema Formation after Focal Cerebral Ischemia in Rats
- Minoxidil Regulates Aging-Like Phenotypes in Rat Cortical Astrocytes In Vitro
- Transcriptional Upregulation of Plasminogen Activator Inhibitor-1 in Rat Primary Astrocytes by a Proteasomal Inhibitor MG132
- Effect of glial-neuronal cell co-culture on GFAP expression of astrocytes
- Astrocytes, Microglia, and Parkinson's Disease