J Stroke.
2021 Sep;23(3):343-357. 10.5853/jos.2021.02446.
Hemodynamics of Leptomeningeal Collaterals after Large Vessel Occlusion and Blood Pressure Management with Endovascular Treatment
- Affiliations
-
- 1Department of Neurology and Cerebrovascular Center, Seoul National University Bundang Hospital, Seongnam, Korea
- 2Department of Clinical Neurosciences, Foothills Medical Center, University of Calgary, Calgary, AB, Canada
- KMID: 2520912
- DOI: http://doi.org/10.5853/jos.2021.02446
Abstract
- Endovascular therapy (EVT) is an effective treatment for ischemic stroke due to large vessel occlusion (LVO). Unlike intravenous thrombolysis, EVT enables visualization of the restoration of blood flow, also known as successful reperfusion in real time. However, until successful reperfusion is achieved, the survival of the ischemic brain is mainly dependent on blood flow from the leptomeningeal collaterals (LMC). It plays a critical role in maintaining tissue perfusion after LVO via pre-existing channels between the arborizing pial small arteries or arterioles overlying the cerebral hemispheres. In the ischemic territory where the physiologic cerebral autoregulation is impaired and the pial arteries are maximally dilated within their capacity, the direction and amount of LMC perfusion rely on the systemic perfusion, which can be estimated by measuring blood pressure (BP). After the EVT procedure, treatment focuses on mitigating the risk of hemorrhagic transformation, potentially via BP reduction. Thus, BP management may be a key component of acute care for patients with LVO stroke. However, the guidelines on BP management during and after EVT are limited, mostly due to the scarcity of high-level evidence on this issue. In this review, we aim to summarize the anatomical and physiological characteristics of LMC to maintain cerebral perfusion after acute LVO, along with a landscape summary of the literature on BP management in endovascular treatment. The objective of this review is to describe the mechanistic association between systemic BP and collateral perfusion after LVO and thus provide clinical and research perspectives on this topic.
Keyword
Figure
Cited by 1 articles
-
Collateral Circulation in Ischemic Stroke: An Updated Review
Gino Maguida, Ashfaq Shuaib
J Stroke. 2023;25(2):179-198. doi: 10.5853/jos.2022.02936.
Reference
-
References
1. Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016; 387:1723–1731.
Article2. Yu I, Bang OY, Chung JW, Kim YC, Choi EH, Seo WK, et al. Admission diffusion-weighted imaging lesion volume in patients with large vessel occlusion stroke and Alberta Stroke Program Early CT Score of ≥6 points: serial computed tomography-magnetic resonance imaging collateral measurements. Stroke. 2019; 50:3115–3120.3. Albers GW. Late window paradox. Stroke. 2018; 49:768–771.
Article4. Winship IR. Cerebral collaterals and collateral therapeutics for acute ischemic stroke. Microcirculation. 2015; 22:228–236.
Article5. Brozici M, van der Zwan A, Hillen B. Anatomy and functionality of leptomeningeal anastomoses: a review. Stroke. 2003; 34:2750–2762.6. Cipolla MJ, McCall AL, Lessov N, Porter JM. Reperfusion decreases myogenic reactivity and alters middle cerebral artery function after focal cerebral ischemia in rats. Stroke. 1997; 28:176–180.
Article7. Gibo H, Carver CC, Rhoton AL Jr, Lenkey C, Mitchell RJ. Microsurgical anatomy of the middle cerebral artery. J Neurosurg. 1981; 54:151–169.
Article8. Gomes FB, Dujovny M, Umansky F, Berman SK, Diaz FG, Ausman JI, et al. Microanatomy of the anterior cerebral artery. Surg Neurol. 1986; 26:129–141.
Article9. Zarrinkoob L, Ambarki K, Wåhlin A, Birgander R, Eklund A, Malm J. Blood flow distribution in cerebral arteries. J Cereb Blood Flow Metab. 2015; 35:648–654.
Article10. Jones EG. On the mode of entry of blood vessels into the cerebral cortex. J Anat. 1970; 106(Pt 3):507–520.11. Duvernoy HM, Delon S, Vannson JL. Cortical blood vessels of the human brain. Brain Res Bull. 1981; 7:519–579.
Article12. Kennedy McConnell F, Payne S. The dual role of cerebral autoregulation and collateral flow in the circle of willis after major vessel occlusion. IEEE Trans Biomed Eng. 2017; 64:1793–1802.
Article13. Aaslid R, Lindegaard KF, Sorteberg W, Nornes H. Cerebral autoregulation dynamics in humans. Stroke. 1989; 20:45–52.
Article14. Ainslie PN, Duffin J. Integration of cerebrovascular CO2 reactivity and chemoreflex control of breathing: mechanisms of regulation, measurement, and interpretation. Am J Physiol Regul Integr Comp Physiol. 2009; 296:R1473–R1495.15. Peterson EC, Wang Z, Britz G. Regulation of cerebral blood flow. Int J Vasc Med. 2011; 2011:823525.
Article16. Asgari S, Bergsneider M, Hamilton R, Vespa P, Hu X. Consistent changes in intracranial pressure waveform morphology induced by acute hypercapnic cerebral vasodilatation. Neurocrit Care. 2011; 15:55–62.
Article17. Witthoft A, Em Karniadakis G. A bidirectional model for communication in the neurovascular unit. J Theor Biol. 2012; 311:80–93.
Article18. Attwell D, Mishra A, Hall CN, O’Farrell FM, Dalkara T. What is a pericyte? J Cereb Blood Flow Metab. 2016; 36:451–455.
Article19. Ma J, Ma Y, Shuaib A, Winship IR. Impaired collateral flow in pial arterioles of aged rats during ischemic stroke. Transl Stroke Res. 2020; 11:243–253.
Article20. Chan SL, Sweet JG, Bishop N, Cipolla MJ. Pial collateral reactivity during hypertension and aging: understanding the function of collaterals for stroke therapy. Stroke. 2016; 47:1618–1625.21. Biose IJ, Dewar D, Macrae IM, McCabe C. Impact of stroke co-morbidities on cortical collateral flow following ischaemic stroke. J Cereb Blood Flow Metab. 2020; 40:978–990.
Article22. Castro P, Azevedo E, Sorond F. Cerebral autoregulation in stroke. Curr Atheroscler Rep. 2018; 20:37.
Article23. Hecht N, Schrammel M, Neumann K, Müller MM, Dreier JP, Vajkoczy P, et al. Perfusion-dependent cerebral autoregulation impairment in hemispheric stroke. Ann Neurol. 2021; 89:358–368.
Article24. Coyle P. Diameter and length changes in cerebral collaterals after middle cerebral artery occlusion in the young rat. Anat Rec. 1984; 210:357–364.
Article25. Morita Y, Fukuuchi Y, Koto A, Suzuki N, Isozumi K, Gotoh J, et al. Rapid changes in pial arterial diameter and cerebral blood flow caused by ipsilateral carotid artery occlusion in rats. Keio J Med. 1997; 46:120–127.
Article26. Rocha M, Jovin TG. Fast versus slow progressors of infarct growth in large vessel occlusion stroke: clinical and research implications. Stroke. 2017; 48:2621–2627.27. Dirnagl U, Pulsinelli W. Autoregulation of cerebral blood flow in experimental focal brain ischemia. J Cereb Blood Flow Metab. 1990; 10:327–336.
Article28. Sandset EC, Anderson CS, Bath PM, Christensen H, Fischer U, Gąsecki D, et al. European Stroke Organisation (ESO) guidelines on blood pressure management in acute ischaemic stroke and intracerebral haemorrhage. Eur Stroke J. 2021; 6:48–89.
Article29. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019; 50:e344–e418.
Article30. Jeong HG, Kim BJ, Kim H, Jung C, Han MK, Liebeskind DS, et al. Blood pressure drop and penumbral tissue loss in nonrecanalized emergent large vessel occlusion. Stroke. 2019; 50:2677–2684.
Article31. Puhr-Westerheide D, Tiedt S, Rotkopf LT, Herzberg M, Reidler P, Fabritius MP, et al. Clinical and imaging parameters associated with hyperacute infarction growth in large vessel occlusion stroke. Stroke. 2019; 50:2799–2804.
Article32. Broocks G, Rajput F, Hanning U, Faizy TD, Leischner H, Schön G, et al. Highest lesion growth rates in patients with hyperacute stroke. Stroke. 2019; 50:189–192.
Article33. El Amki M, Glück C, Binder N, Middleham W, Wyss MT, Weiss T, et al. Neutrophils obstructing brain capillaries are a major cause of no-reflow in ischemic stroke. Cell Rep. 2020; 33:108260.
Article34. Liebeskind DS, Bracard S, Guillemin F, Jahan R, Jovin TG, Majoie CB, et al. eTICI reperfusion: defining success in endovascular stroke therapy. J Neurointerv Surg. 2019; 11:433–438.
Article35. Mistry EA, Mayer SA, Khatri P. Blood pressure management after mechanical thrombectomy for acute ischemic stroke: a survey of the StrokeNet sites. J Stroke Cerebrovasc Dis. 2018; 27:2474–2478.
Article36. Nogueira RG, Liebeskind DS, Sung G, Duckwiler G, Smith WS; MERCI, et al. Predictors of good clinical outcomes, mortality, and successful revascularization in patients with acute ischemic stroke undergoing thrombectomy: pooled analysis of the Mechanical Embolus Removal in Cerebral Ischemia (MERCI) and Multi MERCI Trials. Stroke. 2009; 40:3777–3783.37. Mulder MJ, Ergezen S, Lingsma HF, Berkhemer OA, Fransen PS, Beumer D, et al. Baseline blood pressure effect on the benefit and safety of intra-arterial treatment in MR CLEAN (Multicenter Randomized Clinical Trial of Endovascular Treatment of Acute Ischemic Stroke in the Netherlands). Stroke. 2017; 48:1869–1876.
Article38. Maïer B, Gory B, Taylor G, Labreuche J, Blanc R, Obadia M, et al. Mortality and disability according to baseline blood pressure in acute ischemic stroke patients treated by thrombectomy: a collaborative pooled analysis. J Am Heart Assoc. 2017; 6:e006484.
Article39. Van den Berg SA, Uniken Venema SM, Mulder MJ, Treurniet KM, Samuels N, Lingsma HF, et al. Admission blood pressure in relation to clinical outcomes and successful reperfusion after endovascular stroke treatment. Stroke. 2020; 51:3205–3214.40. Schönenberger S, Uhlmann L, Ungerer M, Pfaff J, Nagel S, Klose C, et al. Association of blood pressure with short- and long-term functional outcome after stroke thrombectomy: post hoc analysis of the SIESTA trial. Stroke. 2018; 49:1451–1456.41. Goyal N, Tsivgoulis G, Iftikhar S, Khorchid Y, Fawad Ishfaq M, Doss VT, et al. Admission systolic blood pressure and outcomes in large vessel occlusion strokes treated with endovascular treatment. J Neurointerv Surg. 2017; 9:451–454.
Article42. Anadani M, Lapergue B, Blanc R, Kyheng M, Labreuche J, Machaa MB, et al. Admission blood pressure and outcome of endovascular therapy: secondary analysis of ASTER trial. J Stroke Cerebrovasc Dis. 2020; 29:105347.
Article43. Davis MJ, Menon BK, Baghirzada LB, Campos-Herrera CR, Goyal M, Hill MD, et al. Anesthetic management and outcome in patients during endovascular therapy for acute stroke. Anesthesiology. 2012; 116:396–405.
Article44. Jagani M, Brinjikji W, Rabinstein AA, Pasternak JJ, Kallmes DF. Hemodynamics during anesthesia for intra-arterial therapy of acute ischemic stroke. J Neurointerv Surg. 2016; 8:883–888.
Article45. Rasmussen M, Espelund US, Juul N, Yoo AJ, Sørensen LH, Sørensen KE, et al. The influence of blood pressure management on neurological outcome in endovascular therapy for acute ischaemic stroke. Br J Anaesth. 2018; 120:1287–1294.
Article46. Pikija S, Trkulja V, Ramesmayer C, Mutzenbach JS, Killer-Oberpfalzer M, Hecker C, et al. Higher blood pressure during endovascular thrombectomy in anterior circulation stroke is associated with better outcomes. J Stroke. 2018; 20:373–384.
Article47. Petersen NH, Ortega-Gutierrez S, Wang A, Lopez GV, Strander S, Kodali S, et al. Decreases in blood pressure during thrombectomy are associated with larger infarct volumes and worse functional outcome. Stroke. 2019; 50:1797–1804.
Article48. Valent A, Sajadhoussen A, Maier B, Lapergue B, Labeyrie MA, Reiner P, et al. A 10% blood pressure drop from baseline during mechanical thrombectomy for stroke is strongly associated with worse neurological outcomes. J Neurointerv Surg. 2020; 12:363–369.
Article49. Rasmussen M, Schönenberger S, Hendèn PL, Valentin JB, Espelund US, Sørensen LH, et al. Blood pressure thresholds and neurologic outcomes after endovascular therapy for acute ischemic stroke: an analysis of individual patient data from 3 randomized clinical trials. JAMA Neurol. 2020; 77:622–631.50. Samuels N, van de Graaf RA, van den Berg CAL, Nieboer D, Eralp I, Treurniet KM, et al. Blood pressure during endovascular treatment under conscious sedation or local anesthesia. Neurology. 2021; 96:e171–e181.
Article51. Maïer B, Dargazanli C, Bourcier R, Kyheng M, Labreuche J, Mosimann PJ, et al. Effect of steady and dynamic blood pressure parameters during thrombectomy according to the collateral status. Stroke. 2020; 51:1199–1206.
Article52. Petersen NH, Silverman A, Strander SM, Kodali S, Wang A, Sansing LH, et al. Fixed compared with autoregulation-oriented blood pressure thresholds after mechanical thrombectomy for ischemic stroke. Stroke. 2020; 51:914–921.
Article53. Löwhagen Hendén P, Rentzos A, Karlsson JE, Rosengren L, Sundeman H, Reinsfelt B, et al. Hypotension during endovascular treatment of ischemic stroke is a risk factor for poor neurological outcome. Stroke. 2015; 46:2678–2680.
Article54. John S, Hazaa W, Uchino K, Toth G, Bain M, Thebo U, et al. Lower intraprocedural systolic blood pressure predicts good outcome in patients undergoing endovascular therapy for acute ischemic stroke. Interv Neurol. 2016; 4:151–157.
Article55. Whalin MK, Halenda KM, Haussen DC, Rebello LC, Frankel MR, Gershon RY, et al. Even small decreases in blood pressure during conscious sedation affect clinical outcome after stroke thrombectomy: an analysis of hemodynamic thresholds. AJNR Am J Neuroradiol. 2017; 38:294–298.
Article56. Athiraman U, Sultan-Qurraie A, Nair B, Tirschwell DL, Ghodke B, Havenon AD, et al. Endovascular treatment of acute ischemic stroke under general anesthesia: predictors of good outcome. J Neurosurg Anesthesiol. 2018; 30:223–230.
Article57. Treurniet KM, Berkhemer OA, Immink RV, Lingsma HF, Wardvan der Stam VM, Hollmann MW, et al. A decrease in blood pressure is associated with unfavorable outcome in patients undergoing thrombectomy under general anesthesia. J Neurointerv Surg. 2018; 10:107–111.
Article58. Pikija S, Millesi K, Killer-Oberpfalzer M, Mutzenbach JS, Sztriha LK, Füssel MU, et al. Blood pressure characteristics in patients with acute basilar artery occlusion undergoing endovascular thrombectomy. Sci Rep. 2019; 9:13224.
Article59. Fandler-Höfler S, Heschl S, Argüelles-Delgado P, Kneihsl M, Hassler E, Magyar M, et al. Single mean arterial blood pressure drops during stroke thrombectomy under general anaesthesia are associated with poor outcome. J Neurol. 2020; 267:1331–1339.
Article60. Xu C, Lin G, Zhang Z, Jin T, Li N, Mao H, et al. Prolonged duration of blood pressure drops during general anesthesia is associated with worse outcomes after mechanical thrombectomy. Front Neurol. 2021; 12:640841.
Article61. Chen M, Kronsteiner D, Pfaff J, Schieber S, Jäger L, Bendszus M, et al. Hemodynamic status during endovascular stroke treatment: association of blood pressure with functional outcome. Neurocrit Care. 2021 Jun 17 [Epub]. https://doi.org/10.1007/s12028-021-01229-w.
Article62. Südfeld S, Brechnitz S, Wagner JY, Reese PC, Pinnschmidt HO, Reuter DA, et al. Post-induction hypotension and early intraoperative hypotension associated with general anaesthesia. Br J Anaesth. 2017; 119:57–64.
Article63. Schönenberger S, Uhlmann L, Hacke W, Schieber S, Mundiyanapurath S, Purrucker JC, et al. Effect of conscious sedation vs general anesthesia on early neurological improvement among patients with ischemic stroke undergoing endovascular thrombectomy: a randomized clinical trial. JAMA. 2016; 316:1986–1996.64. Simonsen CZ, Yoo AJ, Sørensen LH, Juul N, Johnsen SP, Andersen G, et al. Effect of general anesthesia and conscious sedation during endovascular therapy on infarct growth and clinical outcomes in acute ischemic stroke: a randomized clinical trial. JAMA Neurol. 2018; 75:470–477.65. Goyal N, Tsivgoulis G, Pandhi A, Chang JJ, Dillard K, Ishfaq MF, et al. Blood pressure levels post mechanical thrombectomy and outcomes in large vessel occlusion strokes. Neurology. 2017; 89:540–547.
Article66. Cernik D, Sanak D, Divisova P, Kocher M, Cihlar F, Zapletalova J, et al. Impact of blood pressure levels within first 24 hours after mechanical thrombectomy on clinical outcome in acute ischemic stroke patients. J Neurointerv Surg. 2019; 11:735–739.
Article67. Maier IL, Tsogkas I, Behme D, Bähr M, Knauth M, Psychogios MN, et al. High systolic blood pressure after successful endovascular treatment affects early functional outcome in acute ischemic stroke. Cerebrovasc Dis. 2018; 45:18–25.
Article68. Cheng H, Xu C, Jin X, Chen Y, Zheng X, Shi F, et al. Association of blood pressure at successful recanalization and parenchymal hemorrhage after mechanical thrombectomy with general anesthesia. Front Neurol. 2020; 11:582639.
Article69. Matusevicius M, Cooray C, Bottai M, Mazya M, Tsivgoulis G, Nunes AP, et al. Blood pressure after endovascular thrombectomy: modeling for outcomes based on recanalization status. Stroke. 2020; 51:519–525.70. Mistry EA, Sucharew H, Mistry AM, Mehta T, Arora N, Starosciak AK, et al. Blood Pressure after Endovascular Therapy for Ischemic Stroke (BEST): a multicenter prospective cohort study. Stroke. 2019; 50:3449–3455.71. Bennett AE, Wilder MJ, McNally JS, Wold JJ, Stoddard GJ, Majersik JJ, et al. Increased blood pressure variability after endovascular thrombectomy for acute stroke is associated with worse clinical outcome. J Neurointerv Surg. 2018; 10:823–827.
Article72. Mistry EA, Mehta T, Mistry A, Arora N, Starosciak AK, De Los Rios La Rosa F, et al. Blood pressure variability and neurologic outcome after endovascular thrombectomy: a secondary analysis of the BEST Study. Stroke. 2020; 51:511–518.73. Kim TJ, Park HK, Kim JM, Lee JS, Park SH, Jeong HB, et al. Blood pressure variability and hemorrhagic transformation in patients with successful recanalization after endovascular recanalization therapy: a retrospective observational study. Ann Neurol. 2019; 85:574–581.
Article74. Anadani M, Matusevicius M, Tsivgoulis G, Peeters A, Nunes AP, Mancuso M, et al. Magnitude of blood pressure change and clinical outcomes after thrombectomy in stroke caused by large artery occlusion. Eur J Neurol. 2021; 28:1922–1930.
Article75. Mazighi M, Richard S, Lapergue B, Sibon I, Gory B, Berge J, et al. Safety and efficacy of intensive blood pressure lowering after successful endovascular therapy in acute ischaemic stroke (BP-TARGET): a multicentre, open-label, randomised controlled trial. Lancet Neurol. 2021; 20:265–274.
Article76. Anadani M, Arthur AS, Tsivgoulis G, Simpson KN, Alawieh A, Orabi Y, et al. Blood pressure goals and clinical outcomes after successful endovascular therapy: a multicenter study. Ann Neurol. 2020; 87:830–839.
Article77. Liu D, Nie X, Pan Y, Yan H, Pu Y, Wei Y, et al. Adverse outcomes associated with higher mean blood pressure and greater blood pressure variability immediately after successful embolectomy in those with acute ischemic stroke, and the influence of pretreatment collateral circulation status. J Am Heart Assoc. 2021; 10:e019350.
Article78. Castro P, Ferreira F, Nguyen CK, Payabvash S, Ozan Tan C, Sorond F, et al. Rapid assessment of blood pressure variability and outcome after successful thrombectomy. Stroke. 2021; 52:e531–e535.
Article79. Martins AI, Sargento-Freitas J, Silva F, Jesus-Ribeiro J, Correia I, Gomes JP, et al. Recanalization modulates association between blood pressure and functional outcome in acute ischemic stroke. Stroke. 2016; 47:1571–1576.
Article80. Mistry EA, Mistry AM, Nakawah MO, Khattar NK, Fortuny EM, Cruz AS, et al. Systolic blood pressure within 24 hours after thrombectomy for acute ischemic stroke correlates with outcome. J Am Heart Assoc. 2017; 6:e006167.
Article81. Chang JY, Jeon SB, Lee JH, Kwon OK, Han MK. The relationship between blood pressure variability, recanalization degree, and clinical outcome in large vessel occlusive stroke after an intra-arterial thrombectomy. Cerebrovasc Dis. 2018; 46:279–286.
Article82. Martins AI, Sargento-Freitas J, Jesus-Ribeiro J, Correia I, Cardoso L, Gomes JP, et al. Blood pressure variability in acute ischemic stroke: the role of early recanalization. Eur Neurol. 2018; 80:63–67.
Article83. Chang JY, Jeon SB, Jung C, Gwak DS, Han MK. Postreperfusion blood pressure variability after endovascular thrombectomy affects outcomes in acute ischemic stroke patients with poor collateral circulation. Front Neurol. 2019; 10:346.
Article84. Cho BH, Kim JT, Lee JS, Park MS, Kang KW, Choi KH, et al. Associations of various blood pressure parameters with functional outcomes after endovascular thrombectomy in acute ischaemic stroke. Eur J Neurol. 2019; 26:1019–1027.
Article85. Choi KH, Kim JM, Kim JH, Kim JT, Park MS, Choi SM, et al. Optimal blood pressure after reperfusion therapy in patients with acute ischemic stroke. Sci Rep. 2019; 9:5681.
Article86. Zhang T, Wang X, Wen C, Zhou F, Gao S, Zhang X, et al. Effect of short-term blood pressure variability on functional outcome after intra-arterial treatment in acute stroke patients with large-vessel occlusion. BMC Neurol. 2019; 19:228.
Article87. Anadani M, Arthur AS, Alawieh A, Orabi Y, Alexandrov A, Goyal N, et al. Blood pressure reduction and outcome after endovascular therapy with successful reperfusion: a multicenter study. J Neurointerv Surg. 2020; 12:932–936.
Article88. Anadani M, de Havenon A, Yaghi S, Mehta T, Arora N, Starosciak AK, et al. Blood pressure reduction and outcome after endovascular therapy: a secondary analysis of the BEST study. J Neurointerv Surg. 2021; 13:698–702.
Article89. Chu HJ, Lin CH, Chen CH, Hwang YT, Lee M, Lee CW, et al. Effect of blood pressure parameters on functional independence in patients with acute ischemic stroke in the first 6 hours after endovascular thrombectomy. J Neurointerv Surg. 2020; 12:937–941.
Article90. Carvalho Dias M, Gabriel D, Saraiva M, Campos D, Requena M, García-Tornel Á, et al. Spontaneous systolic blood pressure drop early after mechanical thrombectomy predicts dramatic neurological recovery in ischaemic stroke patients. Eur Stroke J. 2020; 5:362–369.
Article91. Ding X, Xu C, Zhong W, Gong X, Zhou Y, Chen Z, et al. Association of maximal systolic blood pressure with poor outcome in patients with hyperattenuated lesions on immediate NCCT after mechanical thrombectomy. J Neurointerv Surg. 2020; 12:127–131.
Article92. McCarthy DJ, Ayodele M, Luther E, Sheinberg D, Bryant JP, Elwardany O, et al. Prolonged heightened blood pressure following mechanical thrombectomy for acute stroke is associated with worse outcomes. Neurocrit Care. 2020; 32:198–205.
Article93. Gigliotti MJ, Padmanaban V, Richardson A, Simon SD, Church EW, Cockroft KM. Effect of blood pressure management strategies on outcomes in patients with acute ischemic stroke after successful mechanical thrombectomy. World Neurosurg. 2021; 148:e635–e642.
Article94. Han B, Sun X, Liu R, Tong X, Jia B, Mo D, et al. Impact of the perioperative blood pressure on clinical outcome after thrombectomy in acute basilar artery occlusion. J Stroke Cerebrovasc Dis. 2021; 30:105590.
Article95. Huang X, Guo H, Yuan L, Cai Q, Zhang M, Zhang Y, et al. Blood pressure variability and outcomes after mechanical thrombectomy based on the recanalization and collateral status. Ther Adv Neurol Disord. 2021; 14:1756286421997383.
Article96. Malhotra K, Goyal N, Katsanos AH, Filippatou A, Mistry EA, Khatri P, et al. Association of blood pressure with outcomes in acute stroke thrombectomy. Hypertension. 2020; 75:730–739.
Article97. De Havenon A, Bennett A, Stoddard GJ, Smith G, Chung L, O’Donnell S, et al. Determinants of the impact of blood pressure variability on neurological outcome after acute ischaemic stroke. Stroke Vasc Neurol. 2017; 2:1–6.
Article98. Mattle HP, Kappeler L, Arnold M, Fischer U, Nedeltchev K, Remonda L, et al. Blood pressure and vessel recanalization in the first hours after ischemic stroke. Stroke. 2005; 36:264–268.
Article99. Brott T, Lu M, Kothari R, Fagan SC, Frankel M, Grotta JC, et al. Hypertension and its treatment in the NINDS rt-PA stroke trial. Stroke. 1998; 29:1504–1509.
Article100. Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015; 372:1019–1030.101. Jovin TG, Saver JL, Ribo M, Pereira V, Furlan A, Bonafe A, et al. Diffusion-weighted imaging or computerized tomography perfusion assessment with clinical mismatch in the triage of wake up and late presenting strokes undergoing neurointervention with Trevo (DAWN) trial methods. Int J Stroke. 2017; 12:641–652.
Article102. Parati G, Ochoa JE, Lombardi C, Bilo G. Assessment and management of blood-pressure variability. Nat Rev Cardiol. 2013; 10:143–155.
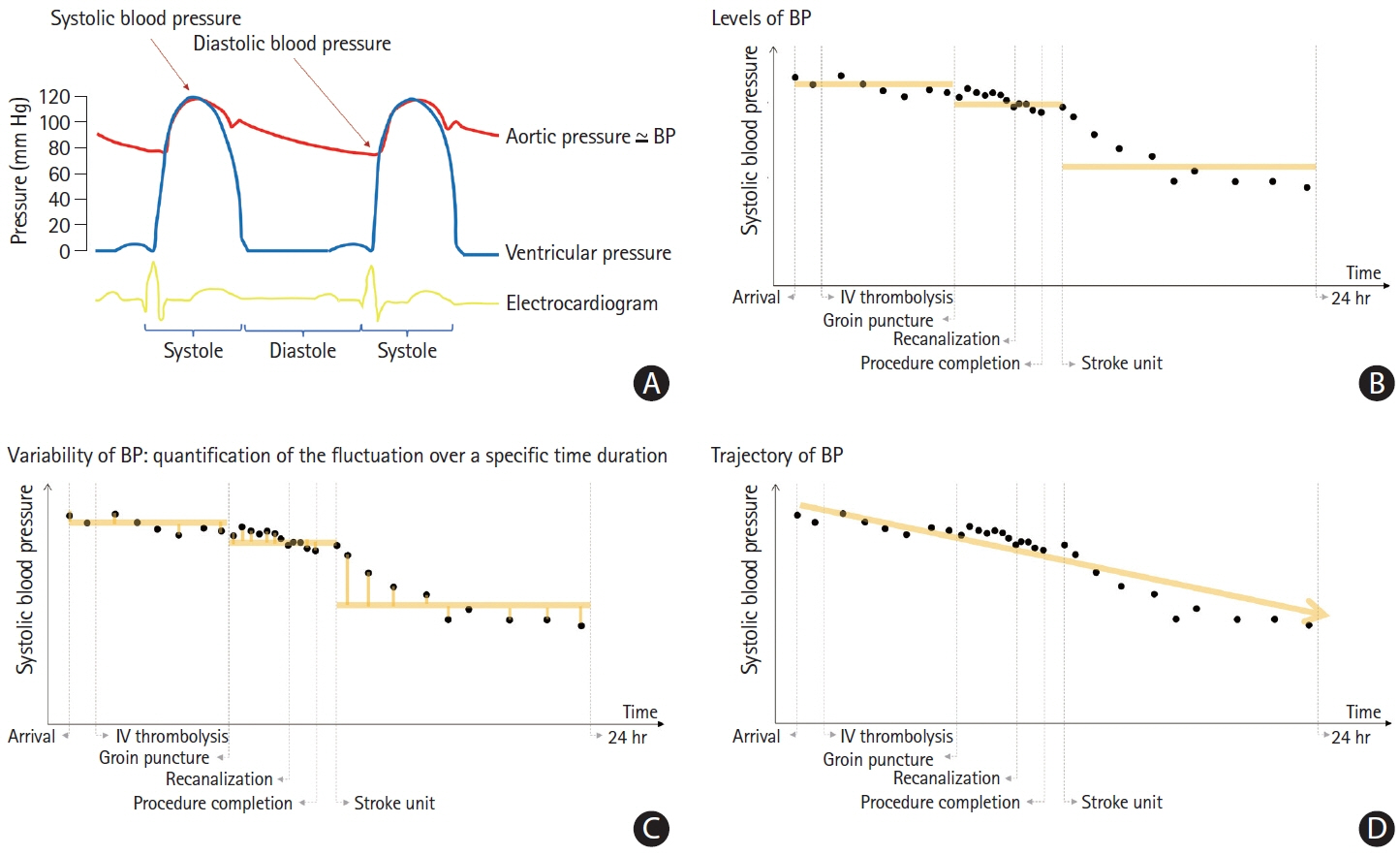
Article103. Kim BJ, Cho YJ, Hong KS, Lee J, Kim JT, Choi KH, et al. Trajectory groups of 24-hour systolic blood pressure after acute ischemic stroke and recurrent vascular events. Stroke. 2018; 49:1836–1842.
Article104. Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol. 2010; 6:109–138.
Article105. Müller M, Österreich M, Lakatos L, Hessling AV. Cerebral macro- and microcirculatory blood flow dynamics in successfully treated chronic hypertensive patients with and without white mater lesions. Sci Rep. 2020; 10:9213.
Article106. Anadani M, de Havenon A, Mistry E, Anderson CS. Blood pressure management after endovascular therapy: an ongoing debate. Stroke. 2021; 52:e263–e265.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hemorrhagic Complications Following Endovascular Treatment for Atherothrombotic Large Vessel Occlusion
- Emergency Microsurgical Embolectomy for the Treatment of Acute Intracranial Artery Occlusion: Report of Two Cases
- Blood Pressure May Be Associated with Arterial Collateralization in Anterior Circulation Ischemic Stroke before Acute Reperfusion Therapy
- Endovascular management of large and giant intracranial aneurysms: Experience from a tertiary care neurosurgery institute in India
- Transcarotid Mechanical Thrombectomy for Embolic Intracranial Large Vessel Occlusion after Endovascular Deconstructice Embolization for Carotid Blowout Syndrome