Maternal Hyperglycemia during Pregnancy Increases Adiposity of Offspring
- Affiliations
-
- 1Department of Pediatrics, Seoul National University Bundang Hospital, Seongnam, Korea
- 2Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea
- 3Department of Pediatrics, Korea Cancer Center Hospital, Seoul, Korea
- 4Department of Pediatrics, Seoul National University Children’s Hospital, Seoul, Korea
- 5Department of Obstetrics and Gynecology, Seoul National University Bundang Hospital, Seongnam, Korea
- 6Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea
- KMID: 2520855
- DOI: http://doi.org/10.4093/dmj.2020.0154
Abstract
- Background
The effect of intrauterine hyperglycemia on fat mass and regional fat proportion of the offspring of mothers with gestational diabetes mellitus (OGDM) remains to be determined.
Methods
The body composition of OGDM (n=25) and offspring of normoglycemic mothers (n=49) was compared using dualenergy X-ray absorptiometry at age 5 years. The relationship between maternal glucose concentration during a 100 g oral glucose tolerance test (OGTT) and regional fat mass or proportion was analyzed after adjusting for maternal prepregnancy body mass index (BMI).
Results
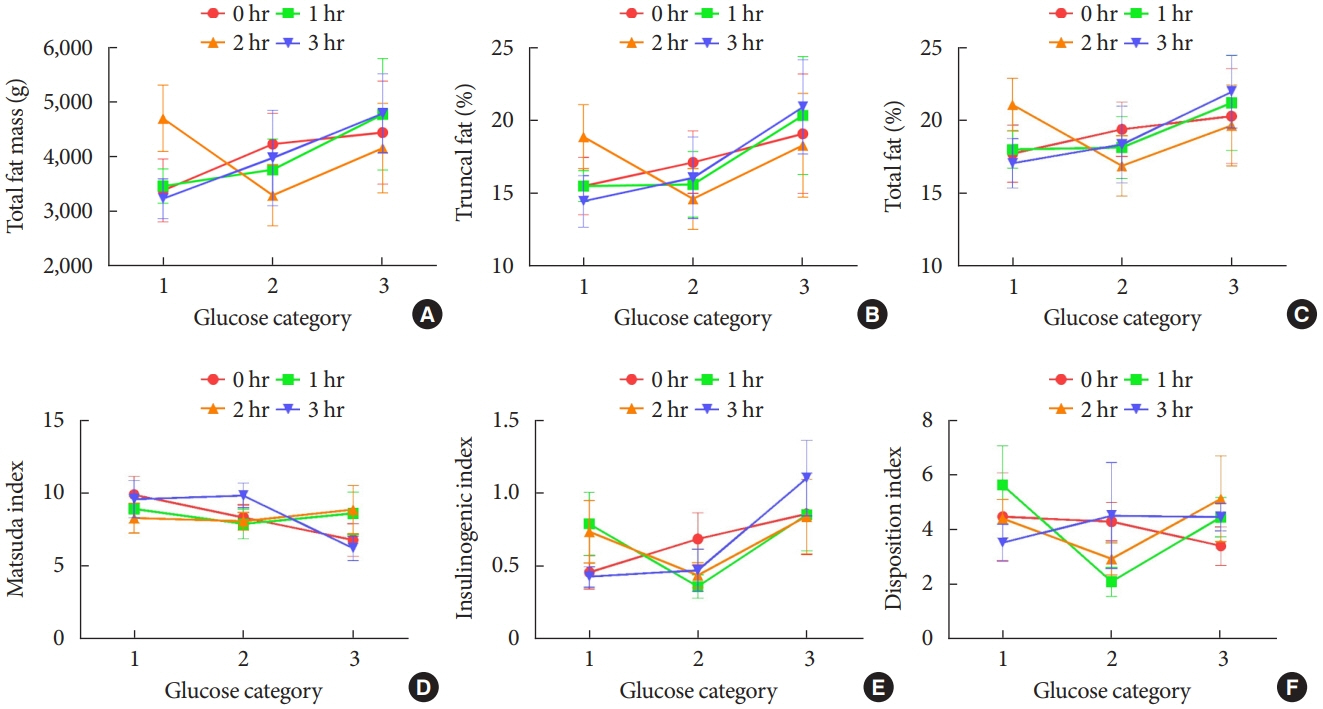
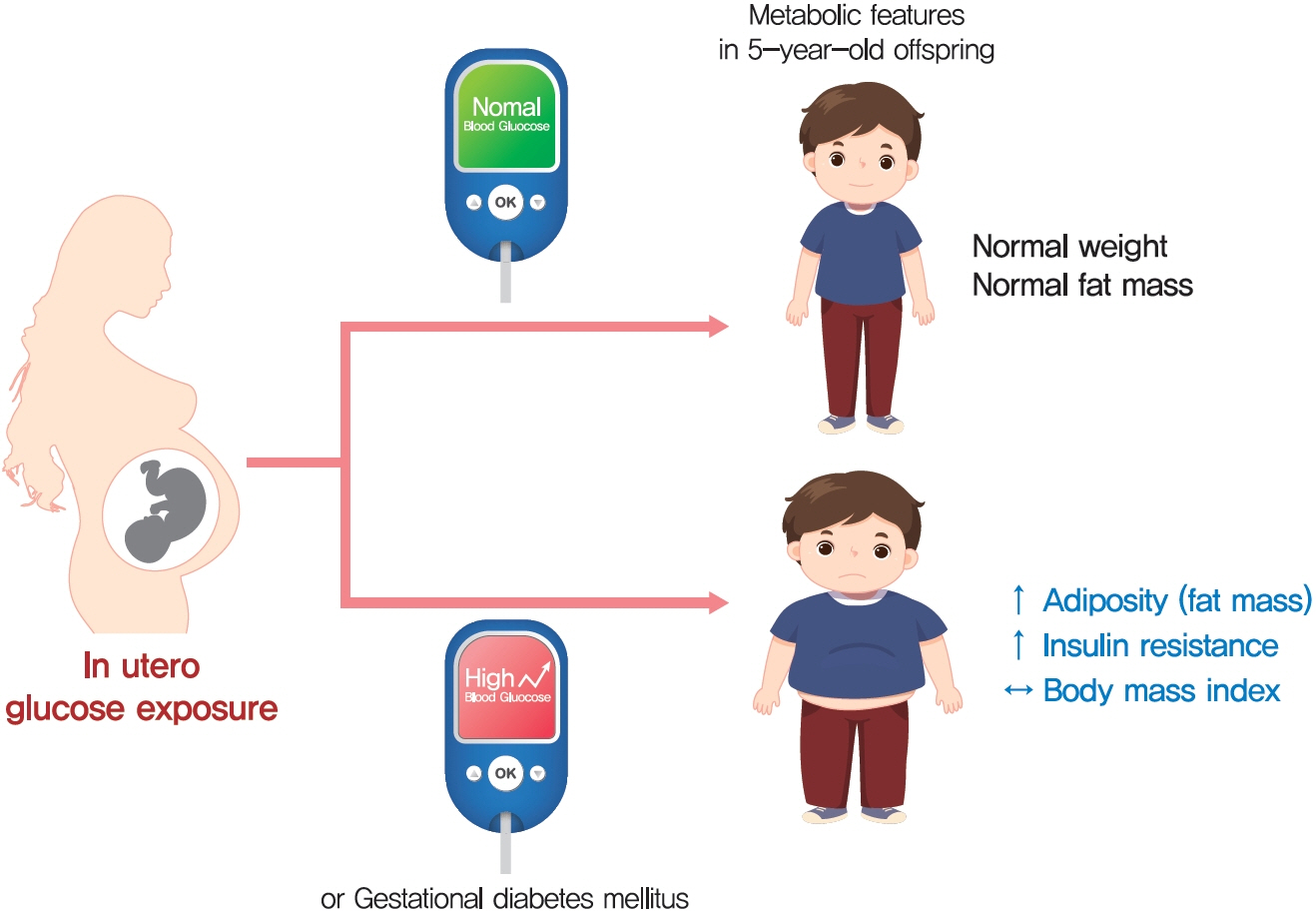
BMI was comparable between OGDM and control (median, 16.0 kg/m2 vs. 16.1 kg/m2 ). Total, truncal, and leg fat mass were higher in OGDM compared with control (3,769 g vs. 2,245 g, P=0.004; 1,289 g vs. 870 g, P=0.017; 1,638 g vs. 961 g, P=0.002, respectively), whereas total lean mass was lower in OGDM (15,688 g vs. 16,941 g, P=0.001). Among OGDM, total and truncal fat mass were correlated with fasting and 3-hour glucose concentrations of maternal 100 g OGTT during pregnancy (total fat mass, r=0.49, P=0.018 [fasting], r=0.473, P=0.023 [3-hour]; truncal fat mass, r=0.571, P=0.004 [fasting], r=0.558, P=0.006 [3-hour]), but there was no correlation between OGDM leg fat mass and maternal OGTT during pregnancy. Regional fat indices were not correlated with concurrent maternal 75 g OGTT values.
Conclusion
Intrauterine hyperglycemia is associated with increased fat mass, especially truncal fat, in OGDM aged 5 years.
Keyword
Figure
Cited by 3 articles
-
Gestational Diabetes Mellitus: Diagnostic Approaches and Maternal-Offspring Complications
Joon Ho Moon, Hak Chul Jang
Diabetes Metab J. 2022;46(1):3-14. doi: 10.4093/dmj.2021.0335.2023 Clinical Practice Guidelines for Diabetes Management in Korea: Full Version Recommendation of the Korean Diabetes Association
Jun Sung Moon, Shinae Kang, Jong Han Choi, Kyung Ae Lee, Joon Ho Moon, Suk Chon, Dae Jung Kim, Hyun Jin Kim, Ji A Seo, Mee Kyoung Kim, Jeong Hyun Lim, Yoon Ju Song, Ye Seul Yang, Jae Hyeon Kim, You-Bin Lee, Junghyun Noh, Kyu Yeon Hur, Jong Suk Park, Sang Youl Rhee, Hae Jin Kim, Hyun Min Kim, Jung Hae Ko, Nam Hoon Kim, Chong Hwa Kim, Jeeyun Ahn, Tae Jung Oh, Soo-Kyung Kim, Jaehyun Kim, Eugene Han, Sang-Man Jin, Jaehyun Bae, Eonju Jeon, Ji Min Kim, Seon Mee Kang, Jung Hwan Park, Jae-Seung Yun, Bong-Soo Cha, Min Kyong Moon, Byung-Wan Lee
Diabetes Metab J. 2024;48(4):546-708. doi: 10.4093/dmj.2024.0249.Gestational Diabetes Mellitus: Mechanisms Underlying Maternal and Fetal Complications
Jooyeop Lee, Na Keum Lee, Joon Ho Moon
Endocrinol Metab. 2025;40(1):10-25. doi: 10.3803/EnM.2024.2264.
Reference
-
1. HAPO Study Cooperative Research Group, Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008; 358:1991–2002.
Article2. Kim MH, Kwak SH, Kim SH, Hong JS, Chung HR, Choi SH, et al. Pregnancy outcomes of women additionally diagnosed as gestational diabetes by the International Association of the Diabetes and Pregnancy Study Groups criteria. Diabetes Metab J. 2019; 43:766–75.
Article3. Freinkel N. Banting lecture 1980. Of pregnancy and progeny. Diabetes. 1980; 29:1023–35.
Article4. Lowe WL Jr, Lowe LP, Kuang A, Catalano PM, Nodzenski M, Talbot O, et al. Maternal glucose levels during pregnancy and childhood adiposity in the Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study. Diabetologia. 2019; 62:598–610.
Article5. Lowe WL Jr, Scholtens DM, Lowe LP, Kuang A, Nodzenski M, Talbot O, et al. Association of gestational diabetes with maternal disorders of glucose metabolism and childhood adiposity. JAMA. 2018; 320:1005–16.
Article6. Wright CS, Rifas-Shiman SL, Rich-Edwards JW, Taveras EM, Gillman MW, Oken E. Intrauterine exposure to gestational diabetes, child adiposity, and blood pressure. Am J Hypertens. 2009; 22:215–20.
Article7. Crume TL, Ogden L, West NA, Vehik KS, Scherzinger A, Daniels S, et al. Association of exposure to diabetes in utero with adiposity and fat distribution in a multiethnic population of youth: the Exploring Perinatal Outcomes among Children (EPOCH) Study. Diabetologia. 2011; 54:87–92.
Article8. Chandler-Laney PC, Bush NC, Granger WM, Rouse DJ, Mancuso MS, Gower BA. Overweight status and intrauterine exposure to gestational diabetes are associated with children’s metabolic health. Pediatr Obes. 2012; 7:44–52.
Article9. Whitaker RC, Pepe MS, Seidel KD, Wright JA, Knopp RH. Gestational diabetes and the risk of offspring obesity. Pediatrics. 1998; 101:E9.
Article10. Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005; 115:e290–6.
Article11. Philipps LH, Santhakumaran S, Gale C, Prior E, Logan KM, Hyde MJ, et al. The diabetic pregnancy and offspring BMI in childhood: a systematic review and meta-analysis. Diabetologia. 2011; 54:1957–66.
Article12. Thaware PK, McKenna S, Patterson CC, Hadden DR, Pettitt DJ, McCance DR. Untreated mild hyperglycemia during pregnancy and anthropometric measures of obesity in offspring at age 5-7 years. Diabetes Care. 2015; 38:1701–6.
Article13. Barlow SE; Expert Committee. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007; 120 Suppl 4:S164–92.
Article14. Cole TJ, Lobstein T. Extended international (IOTF) body mass index cut-offs for thinness, overweight and obesity. Pediatr Obes. 2012; 7:284–94.
Article15. World Medical Association. World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. Bull World Health Organ. 2001; 79:373–4.16. Kim E, Kwak SH, Chung HR, Ohn JH, Bae JH, Choi SH, et al. DNA methylation profiles in sibling pairs discordant for intrauterine exposure to maternal gestational diabetes. Epigenetics. 2017; 12:825–32.
Article17. Lim JS, Hwang JS, Cheon GJ, Lee JA, Kim DH, Park KD, et al. Gender differences in total and regional body composition changes as measured by dual-energy X-ray absorptiometry in Korean children and adolescents. J Clin Densitom. 2009; 12:229–37.
Article18. Moon JH, Kwak SH, Jung HS, Choi SH, Lim S, Cho YM, et al. Weight gain and progression to type 2 diabetes in women with a history of gestational diabetes mellitus. J Clin Endocrinol Metab. 2015; 100:3548–55.
Article19. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2020. Diabetes Care. 2020; 43(Suppl 1):S14–31.20. Moon JS, Lee SY, Nam CM, Choi JM, Choe BK, Seo JW, et al. 2007 Korean national growth charts: review of developmental process and an outlook. Korean J Pediatr. 2008; 51:1–25.
Article21. VanItallie TB, Yang MU, Heymsfield SB, Funk RC, Boileau RA. Height-normalized indices of the body’s fat-free mass and fat mass: potentially useful indicators of nutritional status. Am J Clin Nutr. 1990; 52:953–9.
Article22. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999; 22:1462–70.
Article23. Tura A, Kautzky-Willer A, Pacini G. Insulinogenic indices from insulin and C-peptide: comparison of beta-cell function from OGTT and IVGTT. Diabetes Res Clin Pract. 2006; 72:298–301.
Article24. Retnakaran R, Qi Y, Goran MI, Hamilton JK. Evaluation of proposed oral disposition index measures in relation to the actual disposition index. Diabet Med. 2009; 26:1198–203.
Article25. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985; 28:412–9.26. Chandler-Laney PC, Bush NC, Rouse DJ, Mancuso MS, Gower BA. Maternal glucose concentration during pregnancy predicts fat and lean mass of prepubertal offspring. Diabetes Care. 2011; 34:741–5.
Article27. Unger RH. Minireview: weapons of lean body mass destruction: the role of ectopic lipids in the metabolic syndrome. Endocrinology. 2003; 144:5159–65.
Article28. Laurson KR, Eisenmann JC, Welk GJ. Body fat percentile curves for U. S. children and adolescents. Am J Prev Med. 2011; 41(4 Suppl 2):S87–92.29. Tam WH, Ma RCW, Ozaki R, Li AM, Chan MHM, Yuen LY, et al. In utero exposure to maternal hyperglycemia increases childhood cardiometabolic risk in offspring. Diabetes Care. 2017; 40:679–86.
Article30. Ministry of Health and Welfare: The Third Korea National Health and Nutrition Examination Survey (KNHANES III). Available from: https://knhanes.cdc.go.kr (cited 2021 Jan 12).
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Maternal exposure to airborne particulate matter during pregnancy and lactation induces kidney injury in rat dams and their male offspring: the role of vitamin D in pregnancy and beyond
- Gestational Diabetes Mellitus: Diagnostic Approaches and Maternal-Offspring Complications
- Autism-like Behaviors in Male Juvenile Offspring after Maternal Glyphosate Exposure
- The Maternal Obesity: Adverse Outcomes of Pregnancy and the Offspring
- Maternal high-fructose intake during pregnancy and lactation induces metabolic syndrome in adult offspring