Nutr Res Pract.
2021 Oct;15(5):555-567. 10.4162/nrp.2021.15.5.555.
Inhibitory effect of ethanolic extract of Abeliophyllum distichum leaf on 3T3–L1 adipocyte differentiation
- Affiliations
-
- 1Department of Food Science and Human Nutrition, Jeonbuk National University, Jeonju 54896, Korea
- 2Division of Natural Product Research, Korea Prime Pharmacy Co., Ltd., Gwangju 58144, Korea
- 3Obesity Research Center, Jeonbuk National University, Jeonju 54896, Korea
- 4Department of Food and Nutrition, Chungnam National University, Daejeon 34134, Korea
- KMID: 2520658
- DOI: http://doi.org/10.4162/nrp.2021.15.5.555
Abstract
- BACKGROUND/OBJECTIVES
Abeliophyllum distichum is a plant endemic to Korea, containing several beneficial natural compounds. This study investigated the effect of A. distichum leaf extract (ALE) on adipocyte differentiation.
MATERIALS/METHODS
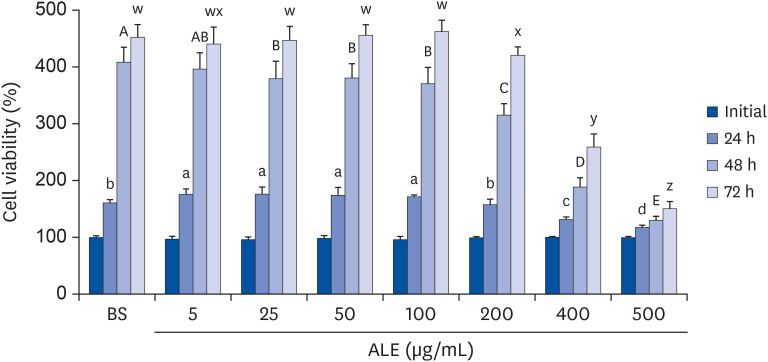
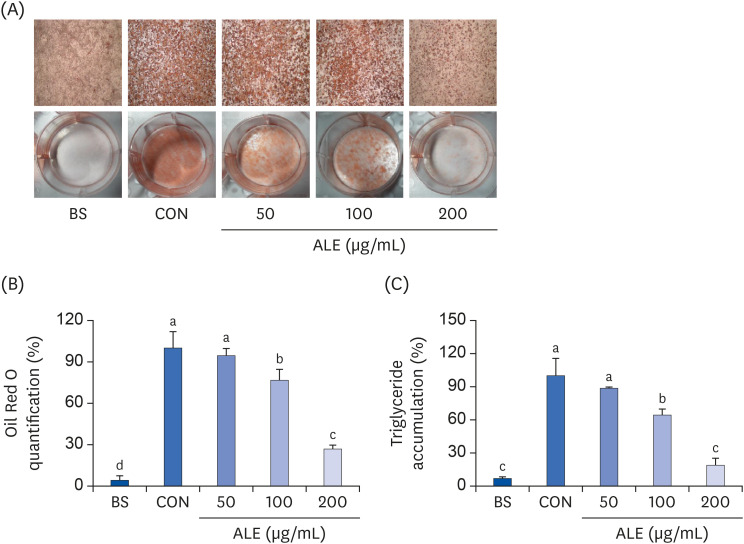
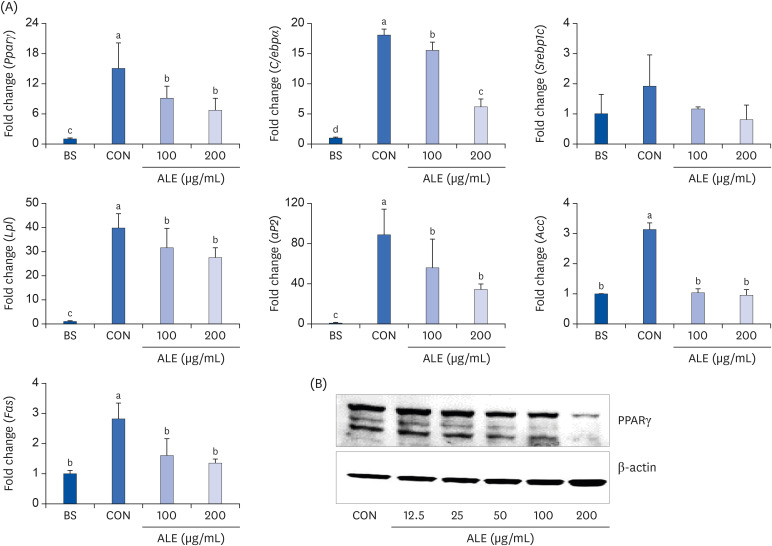
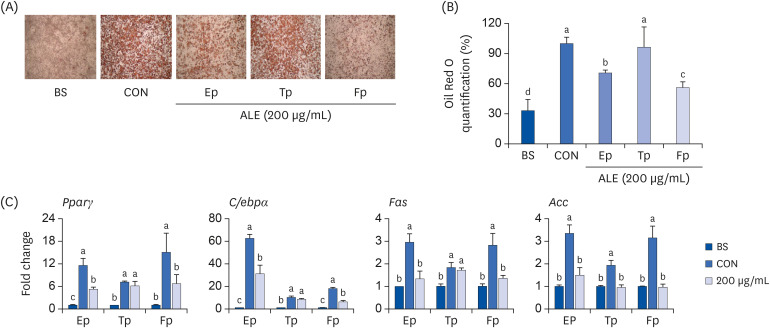
The cytotoxic effect of ALE was analyzed using cell viability assay. 3T3-L1 preadipocytes were differentiated using induction media in the presence or absence of ALE. Lipid accumulation was confirmed using Oil Red O staining. The mRNA expression of adipogenic markers was measured using RT-PCR, and the protein expressions of mitogenactivated protein kinase (MAPK) and peroxisome proliferator-activated receptor gamma (PPARγ) were measured using western blot. Cell proliferation was measured by calculating the incorporation of Bromodeoxyuridine (BrdU) into DNA.
RESULTS
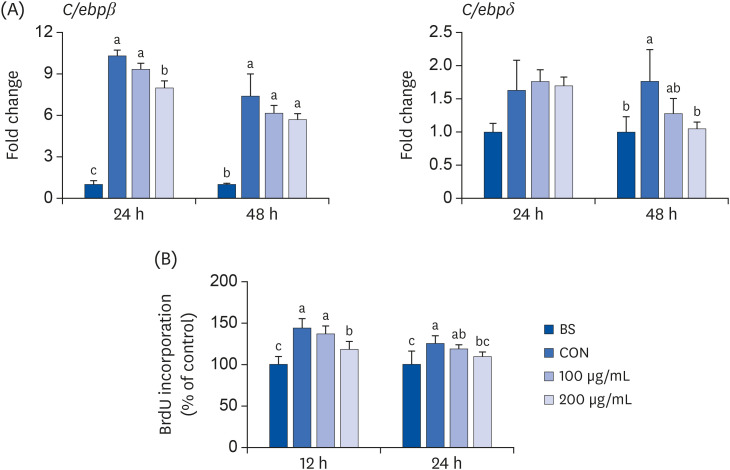
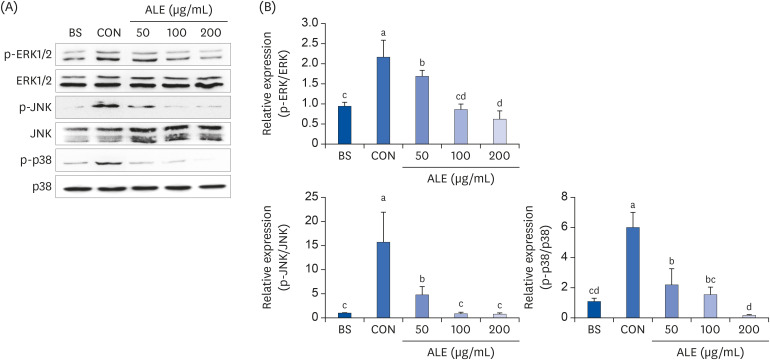
ALE reduced lipid accumulation in differentiated adipocytes, as indicated by Oil Red O staining and triglyceride assays. Treatment with ALE decreased the gene expression of adipogenic markers such as Pparγ, CCAAT/enhancer binding protein alpha (C/ebpα), lipoprotein lipase, adipocyte protein-2, acetyl-CoA carboxylase, and fatty acid synthase. Also, the protein expression of PPARγ was reduced by ALE. Treating the cells with ALE at different time points revealed that the inhibitory effect of ALE on adipogenesis is higher in the early period treatment than in the terminal period. Furthermore, ALE inhibited adipocyte differentiation by reducing the early phase of adipogenesis and mitotic clonal expansion. This was indicated by the lower number of cells in the Synthesis phase of the cell cycle (labeled using BrdU assay) and a decrease in the expression of early adipogenic transcription factors such as C/ebpβ and C/ebpδ. ALE suppressed the phosphorylation of MAPK, confirming that the effect of ALE was through the suppression of early phase of adipogenesis.
CONCLUSIONS
Altogether, the results of the present study revealed that ALE inhibits lipid accumulation and may be a potential agent for managing obesity.
Keyword
Figure
Reference
-
1. Baik I. Forecasting obesity prevalence in Korean adults for the years 2020 and 2030 by the analysis of contributing factors. Nutr Res Pract. 2018; 12:251–257. PMID: 29854331.
Article2. Behl S, Misra A. Management of obesity in adult Asian Indians. Indian Heart J. 2017; 69:539–544. PMID: 28822528.
Article3. Chang E, Kim CY. Natural products and obesity: a focus on the regulation of mitotic clonal expansion during adipogenesis. Molecules. 2019; 24:1157.
Article4. Choe SS, Huh JY, Hwang IJ, Kim JI, Kim JB. Adipose tissue remodeling: its role in energy metabolism and metabolic disorders. Front Endocrinol (Lausanne). 2016; 7:30. PMID: 27148161.
Article5. Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006; 7:885–896. PMID: 17139329.
Article6. Kim CY, Le TT, Chen C, Cheng JX, Kim KH. Curcumin inhibits adipocyte differentiation through modulation of mitotic clonal expansion. J Nutr Biochem. 2011; 22:910–920. PMID: 21189228.
Article7. Moseti D, Regassa A, Kim WK. Molecular regulation of adipogenesis and potential anti-adipogenic bioactive molecules. Int J Mol Sci. 2016; 17:124.
Article8. Ha JH, Jang J, Chung SI, Yoon Y. AMPK and SREBP-1c mediate the anti-adipogenic effect of β-hydroxyisovalerylshikonin. Int J Mol Med. 2016; 37:816–824. PMID: 26865314.
Article9. Thomas SS, Kim M, Lee SJ, Cha YS. Antiobesity effects of purple perilla (perilla frutescens var. acuta) on adipocyte differentiation and mice fed a high‐fat diet. J Food Sci. 2018; 83:2384–2393. PMID: 30070698.10. Lee JW, Kang YJ. Anti-inflammatory effects of Abeliophyllum distichum flower extract and associated MAPKs and NF-κB pathway in raw264.7 cells. Korean J Plant Resour. 2018; 31:202–210.11. Jang TW, Park JH. Antioxidant activity and inhibitory effects on oxidative DNA damage of callus from Abeliophyllum distichum Nakai. Korean J Plant Resour. 2018; 31:228–236.12. Park GH, Park JH, Eo HJ, Song HM, Woo SH, Kim MK, Lee JW, Lee MH, Lee JR, Koo JS, Jeong JB. The induction of activating transcription factor 3 (ATF3) contributes to anti-cancer activity of Abeliophyllum distichum Nakai in human colorectal cancer cells. BMC Complement Altern Med. 2014; 14:487. PMID: 25494848.
Article13. Kim EY, Kim JH, Kim M, Park JH, Sohn Y, Jung HS. Abeliophyllum distichum Nakai alleviates postmenopausal osteoporosis in ovariectomized rats and prevents RANKL-induced osteoclastogenesis in vitro. J Ethnopharmacol. 2020; 257:112828. PMID: 32268206.
Article14. Eom J, Thomas SS, Sung NY, Kim DS, Cha YS, Kim KA. Abeliophyllum distichum ameliorates high-fat diet-induced obesity in C57BL/6J mice by upregulating the AMPK pathway. Nutrients. 2020; 12:3320.15. Kim M, Park JE, Song SB, Cha YS. Effects of black adzuki bean (Vigna angularis) extract on proliferation and differentiation of 3T3-L1 preadipocytes into mature adipocytes. Nutrients. 2015; 7:277–292. PMID: 25569623.
Article16. Gwon SY, Ahn JY, Jung CH, Moon BK, Ha TY. Shikonin suppresses ERK 1/2 phosphorylation during the early stages of adipocyte differentiation in 3T3-L1 cells. BMC Complement Altern Med. 2013; 13:207. PMID: 23919458.
Article17. Stern JH, Rutkowski JM, Scherer PE. Adiponectin, leptin, and fatty acids in the maintenance of metabolic homeostasis through adipose tissue crosstalk. Cell Metab. 2016; 23:770–784. PMID: 27166942.
Article18. Yoo TK, Kim JS, Hyun TK. Polyphenolic composition and anti-melanoma activity of white forsythia (Abeliophyllum distichum nakai) organ extracts. Plants (Basel). 2020; 9:757.
Article19. Sun L, Yu F, Yi F, Xu L, Jiang B, Le L, Xiao P. Acteoside from Ligustrum robustum (Roxb.) blume ameliorates lipid metabolism and synthesis in a HepG2 cell model of lipid accumulation. Front Pharmacol. 2019; 10:602. PMID: 31178740.
Article20. Rosen ED, Hsu CH, Wang X, Sakai S, Freeman MW, Gonzalez FJ, Spiegelman BM. C/EBPalpha induces adipogenesis through PPARgamma: a unified pathway. Genes Dev. 2002; 16:22–26. PMID: 11782441.21. Tang QQ, Otto TC, Lane MD. CCAAT/enhancer-binding protein β is required for mitotic clonal expansion during adipogenesis. Proc Natl Acad Sci U S A. 2003; 100:850–855. PMID: 12525691.
Article22. Hishida T, Nishizuka M, Osada S, Imagawa M. The role of C/EBPdelta in the early stages of adipogenesis. Biochimie. 2009; 91:654–657. PMID: 19233245.23. Hishida T, Eguchi T, Osada S, Nishizuka M, Imagawa M. A novel gene, fad49, plays a crucial role in the immediate early stage of adipocyte differentiation via involvement in mitotic clonal expansion. FEBS J. 2008; 275:5576–5588. PMID: 18959745.
Article24. Oh JH, Karadeniz F, Lee JI, Seo Y, Kong CS. Artemisia princeps inhibits adipogenic differentiation of 3T3-L1 pre-adipocytes via downregulation of PPARγ and MAPK pathways. Prev Nutr Food Sci. 2019; 24:299–307. PMID: 31608255.
Article25. Pal M, Febbraio MA, Lancaster GI. The roles of c-Jun NH2-terminal kinases (JNKs) in obesity and insulin resistance. J Physiol. 2016; 594:267–279. PMID: 26608096.
Article26. Takenouchi T, Takayama Y, Takezawa T. Co-treatment with dexamethasone and octanoate induces adipogenesis in 3T3-L1 cells. Cell Biol Int. 2004; 28:209–216. PMID: 14984747.
Article27. Nam SY, Kim HY, Yoou MS, Kim AH, Park BJ, Jeong HJ, Kim HM. Anti-inflammatory effects of isoacteoside from Abeliophyllum distichum. Immunopharmacol Immunotoxicol. 2015; 37:258–264. PMID: 25975581.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antioxidant and anti-inflammatory effects and mechanism of Abeliophyllum distichum leaf extract in RAW264.7 macrophages
- Nelumbo nucifera Leaf Extract Regulates Lipid Metabolism and Differentiation in 3T3-L1 Adipocytes and db/db Mice
- Psidium guajava L. leaf extract inhibits adipocyte differentiation and improves insulin sensitivity in 3T3-L1 cells
- Effects of (6)-gingerol, ginger component on adipocyte development and differentiation in 3T3-L1
- Soluble extract of soybean fermented with Aspergillus oryzae GB107 inhibits fat accumulation in cultured 3T3-L1 adipocytes