Korean J Physiol Pharmacol.
2021 Sep;25(5):467-478. 10.4196/kjpp.2021.25.5.467.
Apoptin gene delivery by a PAMAM dendrimer modified with a nuclear localization signal peptide as a gene carrier for brain cancer therapy
- Affiliations
-
- 1Department of Physiology, College of Medicine, Cardiovascular and Metabolic Disease Center, Smart Marine Therapeutics Center, Inje University, Busan 47392, Korea.
- 2Division of Applied Medicine, Research Institute for Korea Medicine, School of Korean Medicine, Pusan National University, Busan 50612, Korea.
- 3Department of Biochemistry, College of Natural Sciences, Chungnam National University, Daejeon 34134, Korea.
- KMID: 2519409
- DOI: http://doi.org/10.4196/kjpp.2021.25.5.467
Abstract
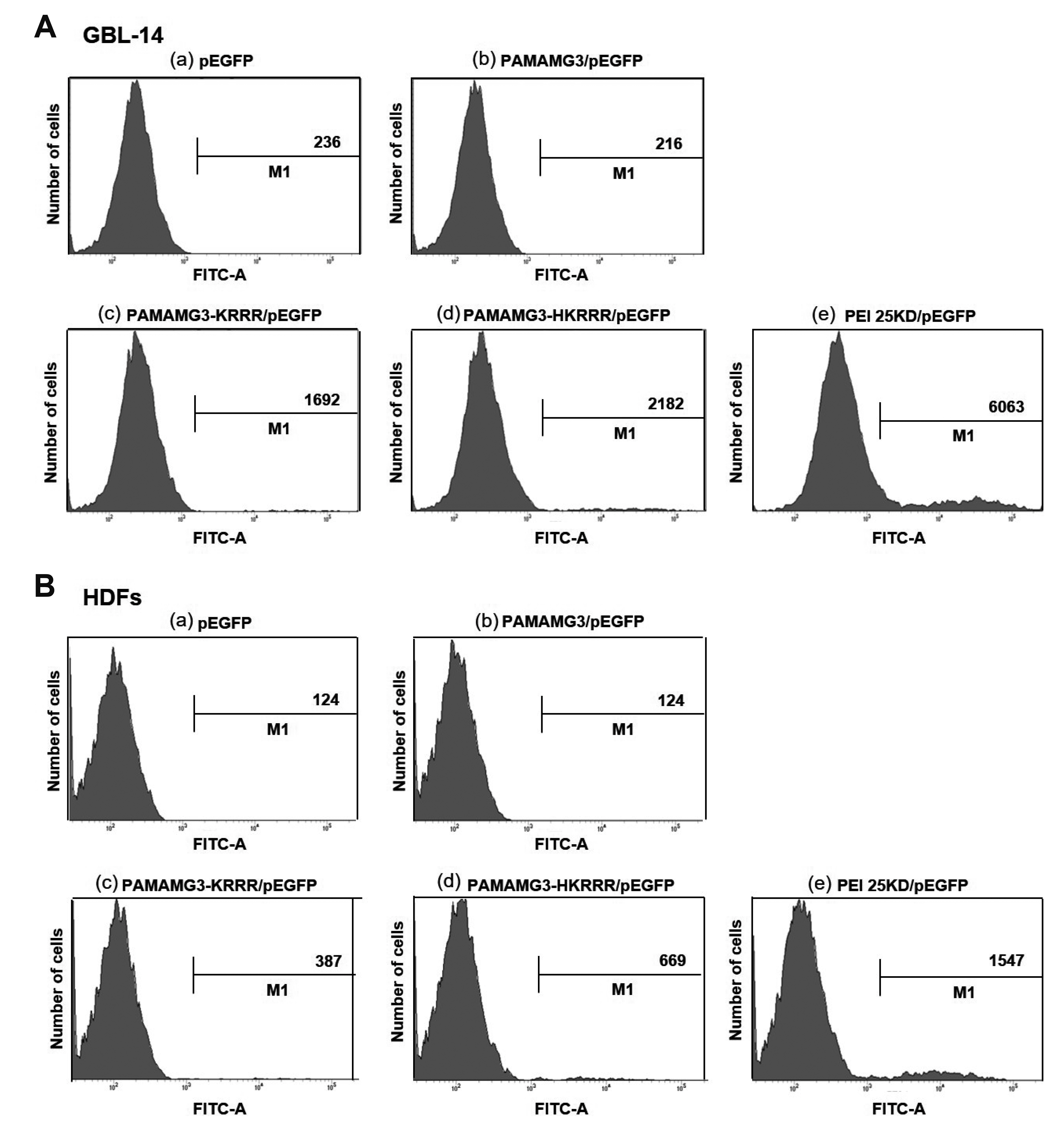
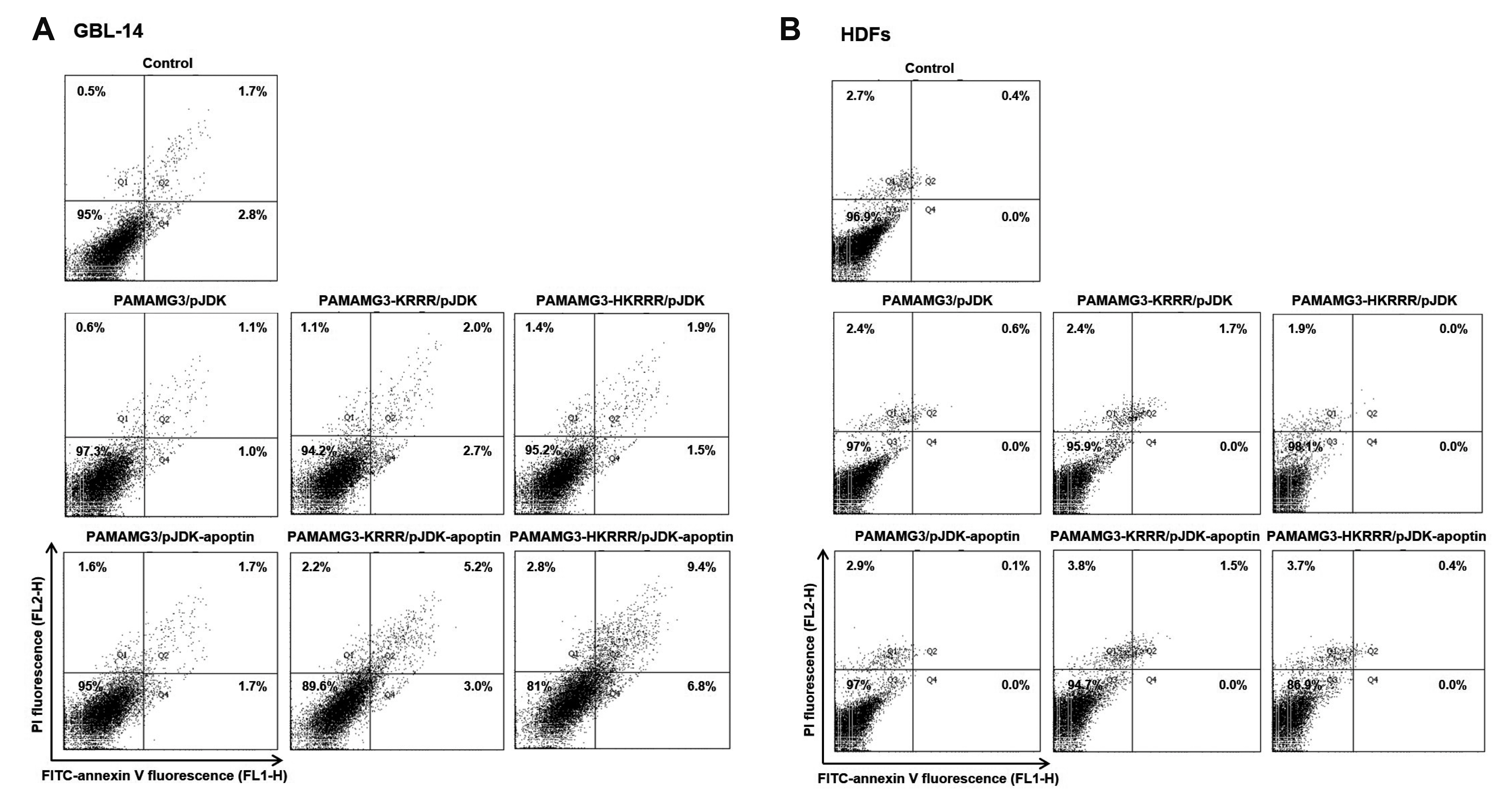
- In this study, we aimed to synthesize PAMAMG3 derivatives (PAMAMG3-KRRR and PAMAMG3-HKRRR), using KRRR peptides as a nuclear localization signal and introduced histidine residues into the KRRR-grafted PAMAMG3 for delivering a therapeutic, carcinoma cell-selective apoptosis gene, apoptin into human primary glioma (GBL-14) cells and human dermal fibroblasts. We examined their cytotoxicity and gene expression using luciferase activity and enhanced green fluorescent protein PAMAMG3 derivatives in both cell lines. We treated cells with PAMAMG3 derivative/apoptin complexes and investigated their intracellular distribution using confocal microscopy. The PAMAMG3-KRRR and PAMAMG3-HKRRR dendrimers were found to escape from endolysosomes into the cytosol. The JC-1 assay, glutathione levels, and Annexin V staining results showed that apoptin triggered cell death in GBL-14 cells. Overall, these findings indicated that the PAMAMG3-HKRRR/apoptin complex is a potential candidate for an effective nonviral gene delivery system for brain tumor therapy in vitro.
Keyword
Figure
Reference
-
1. Zeng W, Tang Z, Li Y, Yin G, Liu Z, Gao J, Chen Y, Chen F. 2020; Patient-derived xenografts of different grade gliomas retain the heterogeneous histological and genetic features of human gliomas. Cancer Cell Int. 20:1. DOI: 10.1186/s12935-019-1086-5. PMID: 31908598. PMCID: PMC6941273.
Article2. Assi H, Candolfi M, Baker G, Mineharu Y, Lowenstein PR, Castro MG. 2012; Gene therapy for brain tumors: basic developments and clinical implementation. Neurosci Lett. 527:71–77. DOI: 10.1016/j.neulet.2012.08.003. PMID: 22906921. PMCID: PMC3462660.
Article3. Reardon DA, Wen PY. 2006; Therapeutic advances in the treatment of glioblastoma: rationale and potential role of targeted agents. Oncologist. 11:152–164. DOI: 10.1634/theoncologist.11-2-152. PMID: 16476836.
Article4. Taylor OG, Brzozowski JS, Skelding KA. 2019; Glioblastoma multiforme: an overview of emerging therapeutic targets. Front Oncol. 9:963. DOI: 10.3389/fonc.2019.00963. PMID: 31616641. PMCID: PMC6775189.
Article5. Pourgholi F, Hajivalili M, Farhad JN, Kafil HS, Yousefi M. 2016; Nanoparticles: novel vehicles in treatment of glioblastoma. Biomed Pharmacother. 77:98–107. DOI: 10.1016/j.biopha.2015.12.014. PMID: 26796272.
Article6. Lin G, Zhang H, Huang L. 2015; Smart polymeric nanoparticles for cancer gene delivery. Mol Pharm. 12:314–321. DOI: 10.1021/mp500656v. PMID: 25531409. PMCID: PMC4319689.
Article7. Balakrishnan B, David E. 2019; Biopolymers augment viral vectors based gene delivery. J Biosci. 44:84. DOI: 10.1007/s12038-019-9905-3. PMID: 31502562.
Article8. Dunbar CE, High KA, Joung JK, Kohn DB, Ozawa K, Sadelain M. 2018; Gene therapy comes of age. Science. 359:eaan4672. DOI: 10.1126/science.aan4672. PMID: 29326244.
Article9. Ramamoorth M, Narvekar A. 2015; Non viral vectors in gene therapy-an overview. J Clin Diagn Res. 9:GE01–GE06. DOI: 10.7860/JCDR/2015/10443.5394. PMID: 25738007. PMCID: PMC4347098.10. Choi YS, Lee MY, David AE, Park YS. 2014; Nanoparticles for gene delivery: therapeutic and toxic effects. Mol Cell Toxicol. 10:1–8. DOI: 10.1007/s13273-014-0001-3.
Article11. Wang LH, Wu T, Wu DC, You YZ. 2016; Bioreducible gene delivery vector capable of self-scavenging the intracellular-generated ROS exhibiting high gene transfection. ACS Appl Mater Interfaces. 8:19238–19244. DOI: 10.1021/acsami.6b04327. PMID: 27420138.
Article12. Nitta SK, Numata K. 2013; Biopolymer-based nanoparticles for drug/gene delivery and tissue engineering. Int J Mol Sci. 14:1629–1654. DOI: 10.3390/ijms14011629. PMID: 23344060. PMCID: PMC3565338.
Article13. Hu J, Zhu M, Liu K, Fan H, Zhao W, Mao Y, Zhang Y. 2016; A biodegradable polyethylenimine-based vector modified by trifunctional peptide R18 for enhancing gene transfection efficiency in vivo. PLoS One. 11:e0166673. DOI: 10.1371/journal.pone.0166673. PMID: 27935984. PMCID: PMC5147860.
Article14. Abbasi E, Aval SF, Akbarzadeh A, Milani M, Nasrabadi HT, Joo SW, Hanifehpour Y, Nejati-Koshki K, Pashaei-Asl R. 2014; Dendrimers: synthesis, applications, and properties. Nanoscale Res Lett. 9:247. DOI: 10.1186/1556-276X-9-247. PMID: 24994950. PMCID: PMC4074873.
Article15. Taghavi PAN, Mutlu P, Khodadust R, Gunduz U. 2013; Poly amidoamine PAMAM nanoparticles: synthesis and biomedical applications. Hacet J Biol Chem. 41:289–299.16. Dutta T, Jain NK, McMillan NA, Parekh HS. 2010; Dendrimer nanocarriers as versatile vectors in gene delivery. Nanomedicine. 6:25–34. DOI: 10.1016/j.nano.2009.05.005. PMID: 19450708.17. Kolhatkar RB, Kitchens KM, Swaan PW, Ghandehari H. 2007; Surface acetylation of polyamidoamine (PAMAM) dendrimers decreases cytotoxicity while maintaining membrane permeability. Bioconjug Chem. 18:2054–2060. DOI: 10.1021/bc0603889. PMID: 17960872.
Article18. Li J, Han Y, Lu Y, Song B, Zhao M, Hu H, Chen D. 2018; A novel disulfide bond-mediated cleavable RGD-modified PAMAM nanocomplex containing nuclear localization signal HMGB1 for enhancing gene transfection efficiency. Int J Nanomedicine. 13:7135–7153. DOI: 10.2147/IJN.S182445. PMID: 30464464. PMCID: PMC6228086.
Article19. Li J, Liang H, Liu J, Wang Z. 2018; Poly (amidoamine) (PAMAM) dendrimer mediated delivery of drug and pDNA/siRNA for cancer therapy. Int J Pharm. 546:215–225. DOI: 10.1016/j.ijpharm.2018.05.045. PMID: 29787895.
Article20. Lee J, Jung J, Kim YJ, Lee E, Choi JS. 2014; Gene delivery of PAMAM dendrimer conjugated with the nuclear localization signal peptide originated from fibroblast growth factor 3. Int J Pharm. 459:10–18. DOI: 10.1016/j.ijpharm.2013.11.027. PMID: 24275448.
Article21. Lee J, Lee S, Kwon YE, Kim YJ, Choi JS. 2019; Gene delivery by PAMAM dendrimer conjugated with the nuclear localization signal peptide derived from influenza B virus nucleoprotein. Macromol Res. 27:360–368. DOI: 10.1007/s13233-019-7057-9.
Article22. Rollano Peñaloza OM, Lewandowska M, Stetefeld J, Ossysek K, Madej M, Bereta J, Sobczak M, Shojaei S, Ghavami S, Łos MJ. 2014; Apoptins: selective anticancer agents. Trends Mol Med. 20:519–528. DOI: 10.1016/j.molmed.2014.07.003. PMID: 25164066.
Article23. Noteborn MH, van der Eb AJ. 1998; Apoptin-induced apoptosis: potential for antitumor therapy. Drug Resist Updat. 1:99–103. DOI: 10.1016/S1368-7646(98)80024-1. PMID: 16904395.
Article24. Maddika S, Booy EP, Johar D, Gibson SB, Ghavami S, Los M. 2005; Cancer-specific toxicity of apoptin is independent of death receptors but involves the loss of mitochondrial membrane potential and the release of mitochondrial cell-death mediators by a Nur77-dependent pathway. J Cell Sci. 118(Pt 19):4485–4493. DOI: 10.1242/jcs.02580. PMID: 16179607.
Article25. Hou Z, Mao J, Lu Y, Li L. 2018; rApoptin induces apoptosis in human breast cancer cells via phosphorylation of Nur77 and Akt. Biochem Biophys Res Commun. 498:221–227. DOI: 10.1016/j.bbrc.2018.02.204. PMID: 29501489.
Article26. An S, Nam K, Choi S, Bai CZ, Lee Y, Park JS. 2013; Nonviral gene therapy in vivo with PAM-RG4/apoptin as a potential brain tumor therapeutic. Int J Nanomedicine. 8:821–834. DOI: 10.2147/IJN.S39072. PMID: 23589689. PMCID: PMC3622651.27. Bae Y, Green ES, Kim GY, Song SJ, Mun JY, Lee S, Park JI, Park JS, Ko KS, Han J, Choi JS. 2016; Dipeptide-functionalized polyamidoamine dendrimer-mediated apoptin gene delivery facilitates apoptosis of human primary glioma cells. Int J Pharm. 515:186–200. DOI: 10.1016/j.ijpharm.2016.09.083. PMID: 27732896.
Article28. Bae Y, Jung MK, Song SJ, Green ES, Lee S, Park HS, Jeong SH, Han J, Mun JY, Ko KS, Choi JS. 2017; Functional nanosome for enhanced mitochondria-targeted gene delivery and expression. Mitochondrion. 37:27–40. DOI: 10.1016/j.mito.2017.06.005. PMID: 28669809.
Article29. Holder AL, Goth-Goldstein R, Lucas D, Koshland CP. 2012; Particle-induced artifacts in the MTT and LDH viability assays. Chem Res Toxicol. 25:1885–1892. DOI: 10.1021/tx3001708. PMID: 22799765. PMCID: PMC3446248.
Article30. Liu BR, Lo SY, Liu CC, Chyan CL, Huang YW, Aronstam RS, Lee HJ. 2013; Endocytic trafficking of nanoparticles delivered by cell-penetrating peptides comprised of nona-arginine and a penetration accelerating sequence. PLoS One. 8:e67100. DOI: 10.1371/journal.pone.0067100. PMID: 23840594. PMCID: PMC3694042.
Article31. Elefantova K, Lakatos B, Kubickova J, Sulova Z, Breier A. 2018; Detection of the mitochondrial membrane potential by the cationic dye JC-1 in L1210 cells with massive overexpression of the plasma membrane ABCB1 drug transporter. Int J Mol Sci. 19:1985. DOI: 10.3390/ijms19071985. PMID: 29986516. PMCID: PMC6073605.
Article32. Dubey A, Goswami M, Yadav K, Chaudhary D. 2015; Oxidative stress and nano-toxicity induced by TiO2 and ZnO on WAG cell line. PLoS One. 10:e0127493. DOI: 10.1371/journal.pone.0127493. PMID: 26011447. PMCID: PMC4444277.
Article33. Uram Ł, Szuster M, Gargasz K, Filipowicz A, Wałajtys-Rode E, Wołowiec S. 2013; In vitro cytotoxicity of the ternary PAMAM G3-pyridoxal-biotin bioconjugate. Int J Nanomedicine. 8:4707–4720. DOI: 10.2147/IJN.S53254. PMID: 24376351. PMCID: PMC3864882.34. Martin ME, Rice KG. 2007; Peptide-guided gene delivery. AAPS J. 9:E18–E29. DOI: 10.1208/aapsj0901003. PMID: 17408236. PMCID: PMC2751301.
Article35. Al-Dosari MS, Gao X. 2009; Nonviral gene delivery: principle, limitations, and recent progress. AAPS J. 11:671–681. DOI: 10.1208/s12248-009-9143-y. PMID: 19834816. PMCID: PMC2782077.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Monitoring Gene Therapy by Radionuclide Approaches
- Modulation of Electroosmotic Flow through Skin: Effect of Poly(Amidoamine) Dendrimers
- The molecular mechanism for nuclear transport and its application
- A immunohistochemical study of localization of calcitonin gene related peptide in the rats cochlear nucleus and superior olivary complex
- Molecular Imaging of Biological Gene Delivery Vehicles for Targeted Cancer Therapy: Beyond Viral Vectors