Korean J Physiol Pharmacol.
2021 Sep;25(5):395-401. 10.4196/kjpp.2021.25.5.395.
Formosanin C attenuates lipopolysaccharide-induced inflammation through nuclear factor-κB inhibition in macrophages
- Affiliations
-
- 1Department of Pharmacy Intravenous Admixture Services, First People's Hospital of Wenling, Wenling 317500, P.R. China.
- 2Department of Rehabilitation Center, First People's Hospital of Wenling, Wenling 317500, P.R. China.
- 3Department of Gastroenterology, First People's Hospital of Wenling, Wenling 317500, P.R. China.
- 4Department of Laboratory Medicine, First People's Hospital of Wenling, Wenling 317500, P.R. China.
- KMID: 2519402
- DOI: http://doi.org/10.4196/kjpp.2021.25.5.395
Abstract
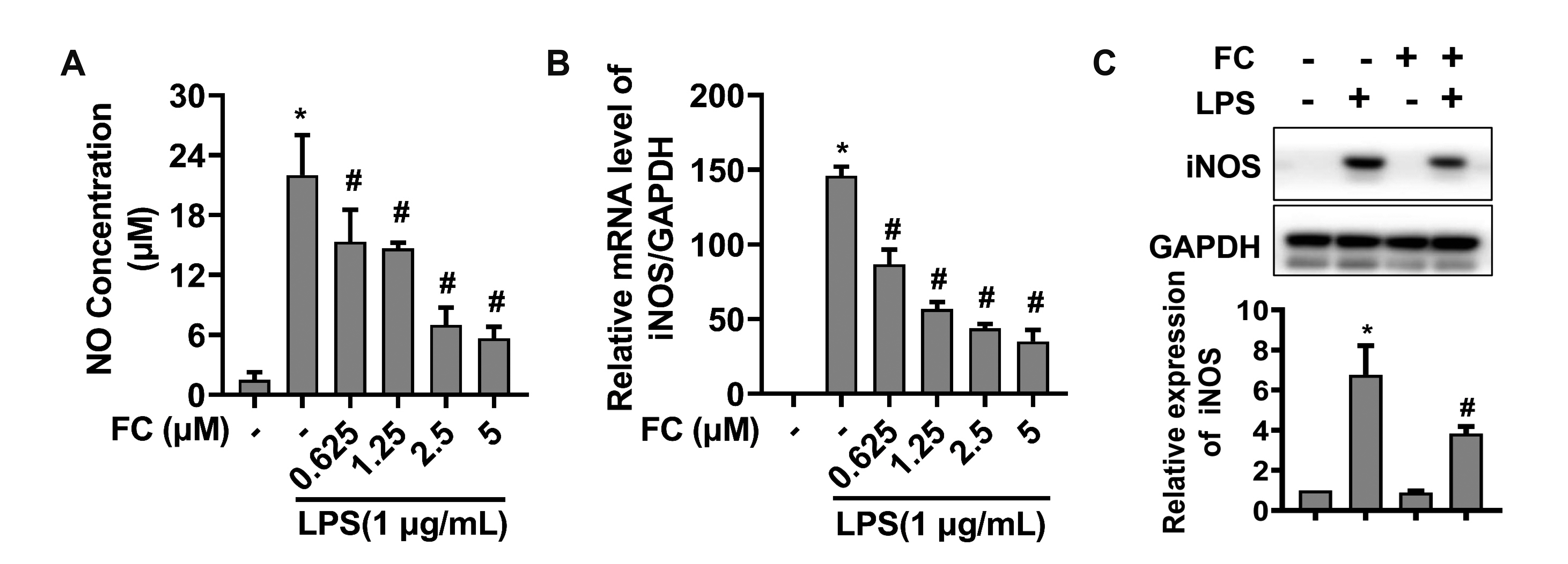
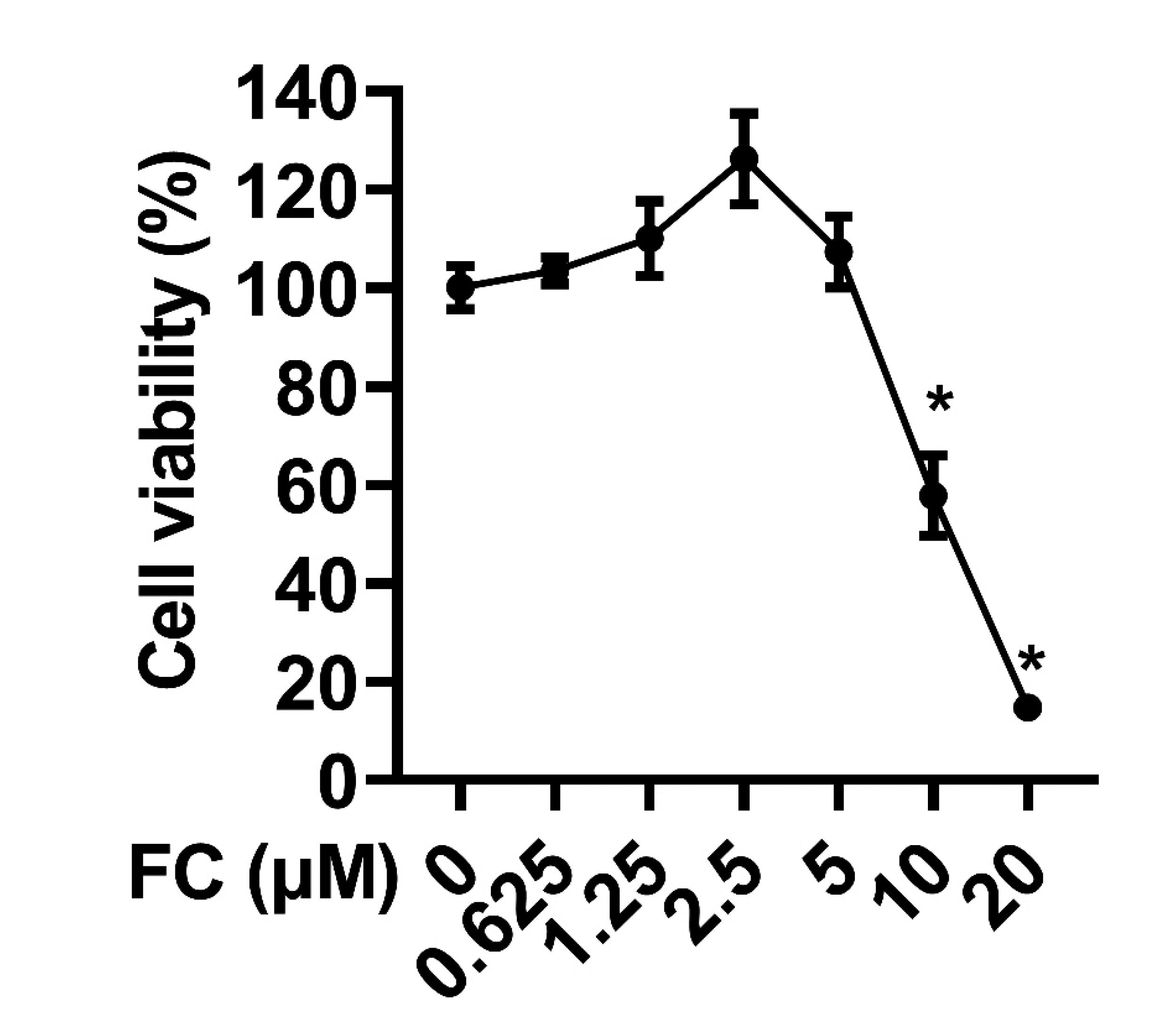
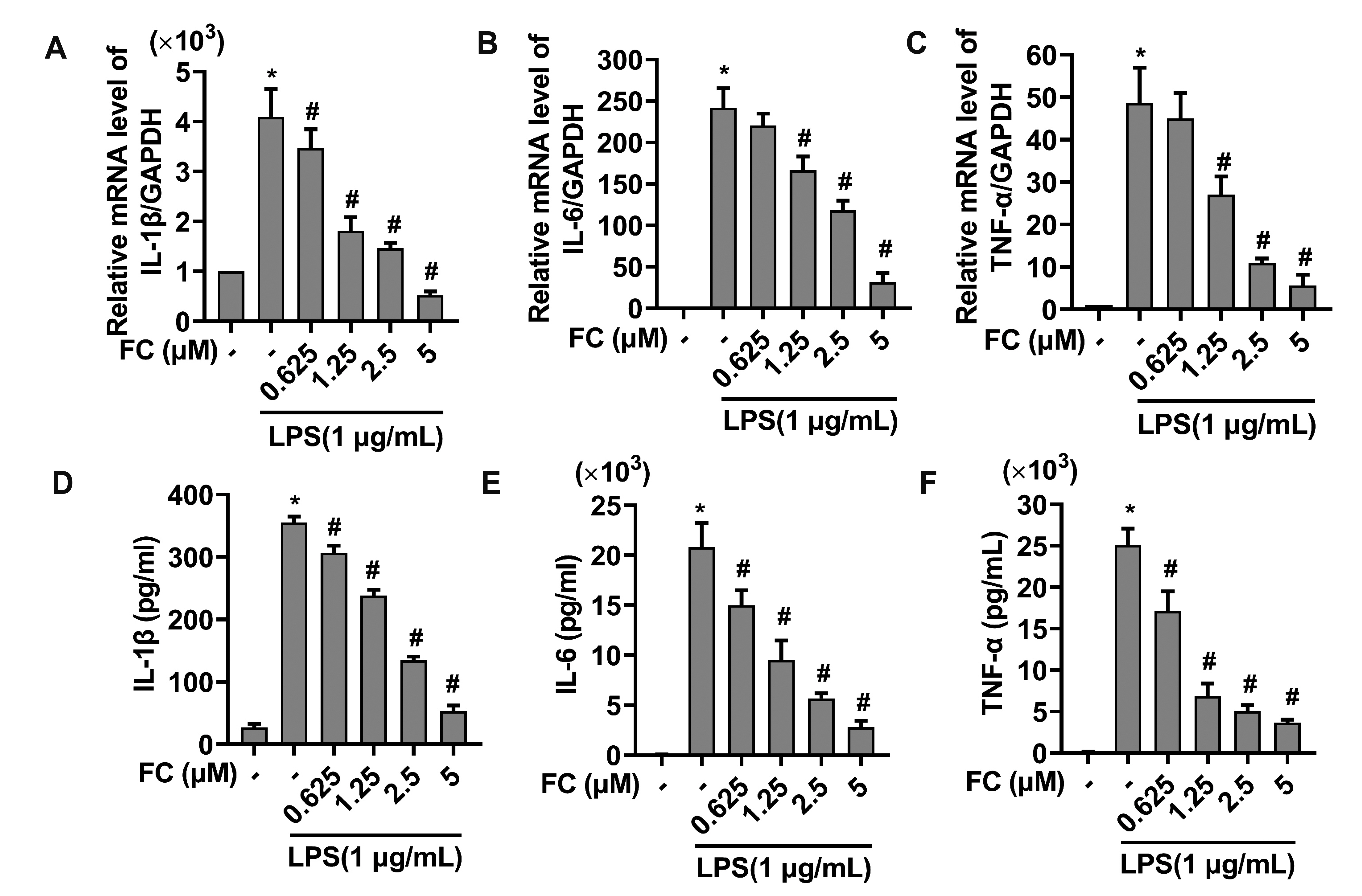
- Extended inflammation and cytokine production pathogenically contribute to a number of inflammatory disorders. Formosanin C (FC) is the major diosgenin saponin found in herb Paris formosana Hayata (Liliaceae), which has been shown to exert anti-cancer and immunomodulatory functions. In this study, we aimed to investigate anti-inflammatory activity of FC and the underlying molecular mechanism. RAW264.7 macrophages were stimulated with lipopolysaccharide (LPS) or pretreated with FC prior to being stimulated with LPS. Thereafter, the macrophages were subjected to analysis of the expression levels of pro-inflammatory mediators, including nitric oxide (NO), prostaglandin E2 (PGE), tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and IL-6, as well as two relevant enzymes, inducible nitric oxide synthase (iNOS), and cyclooxygenase-2 (COX-2). The analysis revealed that FC administration blunted LPS-induced production of NO and PGE in a dose-dependent manner, while the expression of iNOS and COX-2 at both mRNA and protein levels was inhibited in LPS-stimulated macrophages pre-treated with FC. Moreover, LPS stimulation upregulated mRNA expression and medium release of TNF-α, IL-1β, and IL-6, whereas this effect was blocked upon FC pre-administration. Mechanistic studies showed that inhibitory effects of FC on LPS-induced inflammation were associated with a downregulation of IκB kinase, IκB, and p65/NF-κB pathway. Taken together, these data suggest that FC possesses an inflammation-suppressing activity, thus being a potential agent for the treatment of inflammation-associated disorders.
Keyword
Figure
Cited by 1 articles
-
Monotropein resists atherosclerosis by reducing inflammation, oxidative stress, and abnormal proliferation and migration of vascular smooth muscle cells
Hongliang Li, Bingqian Ye, Jiping Tian, Bofan Wang, Yiwen Zha, Shuying Zheng, Tan Ma, Wenwen Zhuang, Won Sun Park, Jingyan Liang
Korean J Physiol Pharmacol. 2025;29(2):245-255. doi: 10.4196/kjpp.24.352.
Reference
-
1. Newton K, Manning G. 2016; Necroptosis and inflammation. Annu Rev Biochem. 85:743–763. DOI: 10.1146/annurev-biochem-060815-014830. PMID: 26865533.
Article2. Bruscia EM, Bonfield TL. 2016; Cystic fibrosis lung immunity: the role of the macrophage. J Innate Immun. 8:550–563. DOI: 10.1159/000446825. PMID: 27336915. PMCID: PMC5089923.
Article3. Liu XH, Pan LL, Jia YL, Wu D, Xiong QH, Wang Y, Zhu YZ. 2013; A novel compound DSC suppresses lipopolysaccharide-induced inflammatory responses by inhibition of Akt/NF-κB signalling in macrophages. Eur J Pharmacol. 708:8–13. DOI: 10.1016/j.ejphar.2013.01.013. PMID: 23353591.
Article4. Moncada S. 1999; Nitric oxide: discovery and impact on clinical medicine. J R Soc Med. 92:164–169. DOI: 10.1177/014107689909200402. PMID: 10450191. PMCID: PMC1297136.
Article5. Miller SI, Ernst RK, Bader MW. 2005; LPS, TLR4 and infectious disease diversity. Nat Rev Microbiol. 3:36–46. DOI: 10.1038/nrmicro1068. PMID: 15608698.
Article6. Viatour P, Merville MP, Bours V, Chariot A. 2005; Phosphorylation of NF-kappaB and IkappaB proteins: implications in cancer and inflammation. Trends Biochem Sci. 30:43–52. DOI: 10.1016/j.tibs.2004.11.009. PMID: 15653325.7. Wu RT, Chiang HC, Fu WC, Chien KY, Chung YM, Horng LY. 1990; Formosanin-C, an immunomodulator with antitumor activity. Int J Immunopharmacol. 12:777–786. DOI: 10.1016/0192-0561(90)90042-L. PMID: 2292459.8. Lee JC, Su CL, Chen LL, Won SJ. 2009; Formosanin C-induced apoptosis requires activation of caspase-2 and change of mitochondrial membrane potential. Cancer Sci. 100:503–513. DOI: 10.1111/j.1349-7006.2008.01057.x. PMID: 19154411.
Article9. Li Y, Man S, Li J, Chai H, Fan W, Liu Z, Gao W. 2014; The antitumor effect of formosanin C on HepG2 cell as revealed by 1H-NMR based metabolic profiling. Chem Biol Interact. 220:193–199. DOI: 10.1016/j.cbi.2014.06.023. PMID: 25014414.
Article10. Man S, Gao W, Zhang Y, Liu Z, Yan L, Huang L, Liu C. 2011; Formosanin C-inhibited pulmonary metastasis through repression of matrix metalloproteinases on mouse lung adenocarcinoma. Cancer Biol Ther. 11:592–598. DOI: 10.4161/cbt.11.6.14668. PMID: 21304274.
Article11. Chiang HC, Wang JJ, Wu RT. 1992; Immunomodulating effects of the hydrolysis products of formosanin C and beta-ecdysone from Paris formosana Hayata. Anticancer Res. 12:1475–1478. PMID: 1444209.12. Cui J, Man S, Cui N, Yang L, Guo Q, Ma L, Gao W. 2019; The synergistic anticancer effect of formosanin C and polyphyllin VII based on caspase-mediated cleavage of Beclin1 inhibiting autophagy and promoting apoptosis. Cell Prolif. 52:e12520. DOI: 10.1111/cpr.12520. PMID: 30338602. PMCID: PMC6430456.
Article13. Liu J, Man S, Liu Z, Ma L, Gao W. 2016; A synergistic antitumor effect of polyphyllin I and formosanin C on hepatocarcinoma cells. Bioorg Med Chem Lett. 26:4970–4975. DOI: 10.1016/j.bmcl.2016.09.005. PMID: 27623551.
Article14. Lin PL, Tang HH, Wu SY, Shaw NS, Su CL. 2020; Saponin formosanin C-induced ferritinophagy and ferroptosis in human hepatocellular carcinoma cells. Antioxidants (Basel). 9:682. DOI: 10.3390/antiox9080682. PMID: 32751249. PMCID: PMC7463707.
Article15. Gu I, Brownmiller C, Stebbins NB, Mauromoustakos A, Howard L, Lee SO. 2020; Berry phenolic and volatile extracts inhibit pro-inflammatory cytokine secretion in LPS-stimulated RAW264.7 cells through suppression of NF-κB signaling pathway. Antioxidants (Basel). 9:871. DOI: 10.3390/antiox9090871. PMID: 32942640. PMCID: PMC7554842.
Article16. Rod-In W, Monmai C, Shin IS, You S, Park WJ. 2020; Neutral lipids, glycolipids, and phospholipids, isolated from sandfish (Arctoscopus japonicus) eggs, exhibit anti-inflammatory activity in LPS-stimulated RAW264.7 cells through NF-κB and MAPKs pathways. Mar Drugs. 18:480. DOI: 10.3390/md18090480. PMID: 32967264. PMCID: PMC7550997.
Article17. Tao MQ, Ji CL, Wu YJ, Dong JY, Li Y, Olatunji OJ, Zuo J. 2020; 1,7-Dihydroxy-3,4-dimethoxyxanthone inhibits lipopolysaccharide-induced inflammation in RAW264.7 macrophages by suppressing TLR4/NF-κB signaling cascades. Inflammation. 43:1821–1831. DOI: 10.1007/s10753-020-01256-3. PMID: 32468498.
Article18. Gao XH, Zhang SD, Wang LT, Yu L, Zhao XL, Ni HY, Wang YQ, Wang JD, Shan CH, Fu YJ. 2020; Anti-inflammatory effects of neochlorogenic acid extract from mulberry leaf (Morus alba L.) against LPS-stimulated inflammatory response through mediating the AMPK/Nrf2 signaling pathway in A549 cells. Molecules. 25:1385. DOI: 10.3390/molecules25061385. PMID: 32197466. PMCID: PMC7144357.19. Wu RT, Lin WJ, Chiang HC, Horng LY, Chung YM. 1990; Modulation of experimental autoimmune uveitis with formosanin-C in guinea pigs. J Ocul Pharmacol. 6:301–311. DOI: 10.1089/jop.1990.6.301. PMID: 2097314.
Article20. Gao H, Liu X, Sun W, Kang N, Liu Y, Yang S, Xu QM, Wang C, Chen X. 2017; Total tanshinones exhibits anti-inflammatory effects through blocking TLR4 dimerization via the MyD88 pathway. Cell Death Dis. 8:e3004. DOI: 10.1038/cddis.2017.389. PMID: 28817116. PMCID: PMC5596575.
Article21. Gao H, Cui Y, Kang N, Liu X, Liu Y, Zou Y, Zhang Z, Li X, Yang S, Li J, Wang C, Xu QM, Chen X. 2017; Isoacteoside, a dihydroxyphenylethyl glycoside, exhibits anti-inflammatory effects through blocking toll-like receptor 4 dimerization. Br J Pharmacol. 174:2880–2896. DOI: 10.1111/bph.13912. PMID: 28616865. PMCID: PMC5554315.
Article22. Chen CC, Lin MW, Liang CJ, Wang SH. 2016; The anti-inflammatory effects and mechanisms of eupafolin in lipopolysaccharide-induced inflammatory responses in RAW264.7 macrophages. PLoS One. 11:e0158662. DOI: 10.1371/journal.pone.0158662. PMID: 27414646. PMCID: PMC4945065.
Article23. Yoo S, Kim MY, Cho JY. 2017; Beauvericin, a cyclic peptide, inhibits inflammatory responses in macrophages by inhibiting the NF-κB pathway. Korean J Physiol Pharmacol. 21:449–456. DOI: 10.4196/kjpp.2017.21.4.449. PMID: 28706459. PMCID: PMC5507784.
Article24. Rossol M, Heine H, Meusch U, Quandt D, Klein C, Sweet MJ, Hauschildt S. 2011; LPS-induced cytokine production in human monocytes and macrophages. Crit Rev Immunol. 31:379–446. DOI: 10.1615/CritRevImmunol.v31.i5.20. PMID: 22142165.
Article25. Oeckinghaus A, Ghosh S. 2009; The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb Perspect Biol. 1:a000034. DOI: 10.1101/cshperspect.a000034. PMID: 20066092. PMCID: PMC2773619.26. Xu Z, Lin R, Hou X, Wu J, Zhao W, Ma H, Fan Z, Li S, Zhu Y, Zhang D. 2020; Immunomodulatory mechanism of a purified polysaccharide isolated from Isaria cicadae Miquel on RAW264.7 cells via activating TLR4-MAPK-NF-κB signaling pathway. Int J Biol Macromol. 164:4329–4338. DOI: 10.1016/j.ijbiomac.2020.09.035. PMID: 32926903.
Article27. Madrid LV, Mayo MW, Reuther JY, Baldwin AS Jr. 2001; Akt stimulates the transactivation potential of the RelA/p65 Subunit of NF-kappa B through utilization of the Ikappa B kinase and activation of the mitogen-activated protein kinase p38. J Biol Chem. 276:18934–18940. DOI: 10.1074/jbc.M101103200. PMID: 11259436.28. Xiao X, Yang M, Xiao J, Zou J, Huang Q, Yang K, Zhang B, Yang F, Liu S, Wang H, Bai P. 2014; Paris Saponin II suppresses the growth of human ovarian cancer xenografts via modulating VEGF-mediated angiogenesis and tumor cell migration. Cancer Chemother Pharmacol. 73:807–818. DOI: 10.1007/s00280-014-2408-x. PMID: 24638862.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anti-inflammatory and immune enhancing activities of PB203 in mouse macrophage RAW264.7 cells
- Galangin Regulates Mucin 5AC Gene Expression via the Nuclear Factor-κB Inhibitor α/Nuclear Factor-κB p65 Pathway in Human Airway Epithelial Cells
- Efonidipine Inhibits JNK and NF-κB Pathway to Attenuate Inflammation and Cell Migration Induced by Lipopolysaccharide in Microglial Cells
- α-Tocopherol and γ-tocopherol decrease inflammatory response and insulin resistance during the interaction of adipocytes and macrophages
- Voluntary Wheel Running Improves Spatial Learning Memory by Suppressing Inflammation and Apoptosis via Inactivation of Nuclear Factor Kappa B in Brain Inflammation Rats