J Rheum Dis.
2021 Jul;28(3):133-142. 10.4078/jrd.2021.28.3.133.
Intravenous Administration of Toll-Like Receptor Inhibitory Peptide 1 is Effective for the Treatment of Systemic Lupus Erythematosus in a Mus musculus Model
- Affiliations
-
- 1Department of Rheumatology, Ajou University School of Medicine, Suwon, Korea
- 2Department of Molecular Science and Technology, Ajou University, Suwon, Korea
- KMID: 2516949
- DOI: http://doi.org/10.4078/jrd.2021.28.3.133
Abstract
Objective
Systemic lupus erythematosus (SLE) is a common chronic autoimmune inflammatory disease. According to recent studies, signaling through Toll-like receptor (TLR) protein, which promotes the production of inflammatory cytokines, leads to the development of SLE. TLR-inhibitory peptide 1 (TIP1) has been newly identified for the treatment of autoimmune diseases.
Methods
The effect of TIP1 was analyzed in an SLE mouse model (MRL/lpr). The mice in the control treatment group (n=5) were administered an intravenous injection of phosphate-buffered saline twice weekly, whereas the mice in the TIP1 treatment group (n=6) were administered an intravenous injection of TIP1 (1 nmol/g) twice weekly. MRL/mpj mice (n=5) were selected as normal controls. The mice were injected for 4 weeks between 14 and 18 weeks of age, followed by assays of their spleen, kidneys, lymph nodes, serum, and urine.
Results
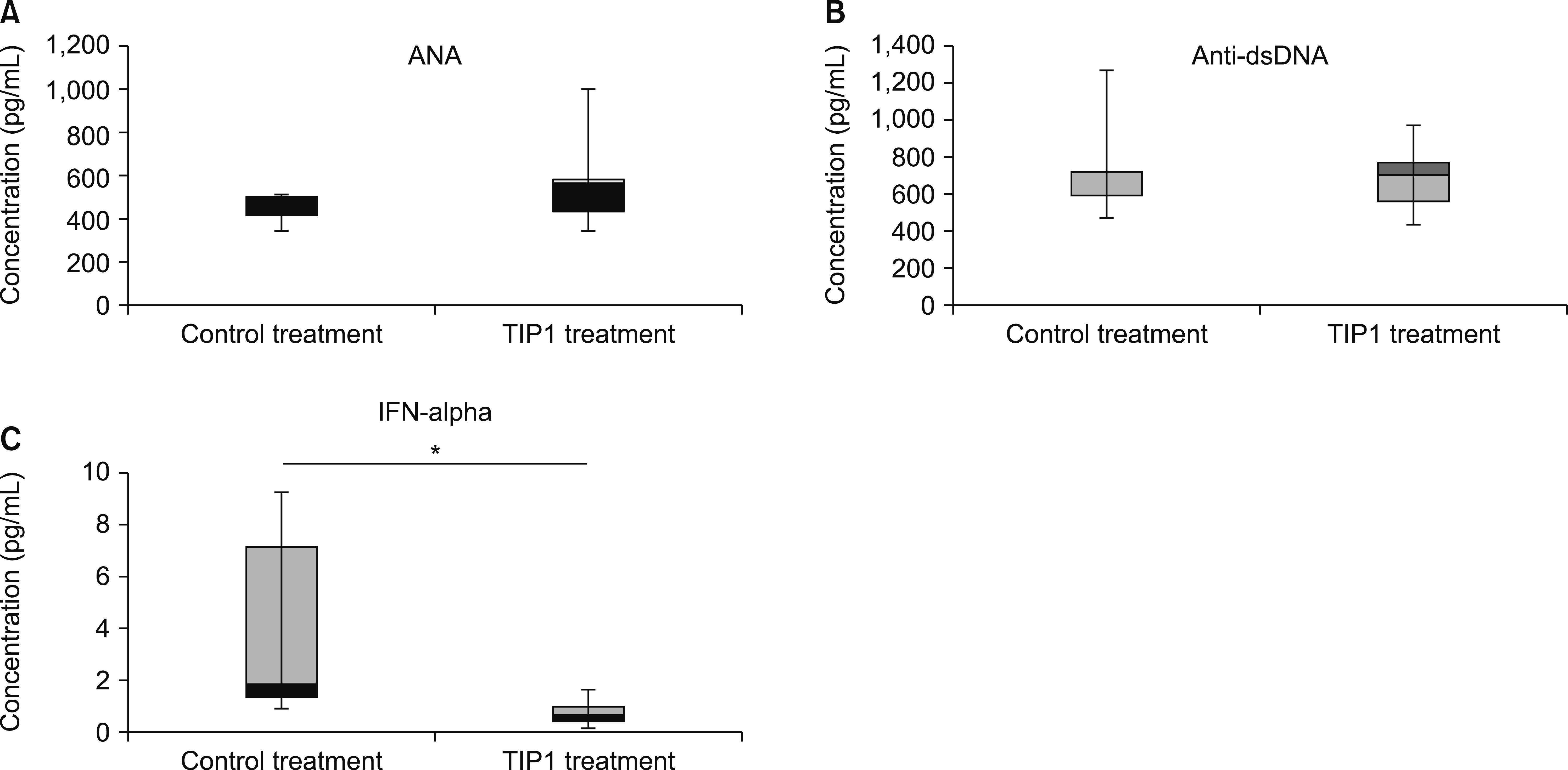
The antinuclear antibody and inflammatory cytokine (interferon-α) in the serum as well as levels of albumin in the urine of the mice in the TIP1 treatment group had decreased when compared to those of mice in the control treatment group. Kidney inflammation in mice in the TIP1 treatment group was alleviated. The mRNA expression levels of TLR7- or TLR9-related downstream signaling molecules also decreased in all organs of the mice in the TIP1 treatment group.
Conclusion
Intravenous treatment with TIP1 reduces symptoms and markers of inflammation in MRL/lpr mice. Hence, TIP1 is a promising medication for the treatment of SLE.
Figure
Reference
-
1. Tsokos GC. 2011; Systemic lupus erythematosus. N Engl J Med. 365:2110–21. DOI: 10.1056/NEJMra1100359. PMID: 22129255.
Article2. Klippel JH. 1997; Systemic lupus erythematosus: demographics, prognosis, and outcome. J Rheumatol Suppl. 48:67–71. PMID: 9150122.3. Petri M. 2001; Long-term outcomes in lupus. Am J Manag Care. 7(16 Suppl):S480–5. PMID: 11680779.4. Toblli JE, Trigo M, Mendoza G, Fellner JP, Genaro O, Houssay R, et al. 1983; Hematologic manifestations in systemic lupus erythematosus. Experience collected from 150 cases. Rev Clin Esp. 169:257–61. Spanish. PMID: 6622769.5. Kamen DL. 2014; Environmental influences on systemic lupus erythematosus expression. Rheum Dis Clin North Am. 40:401–12. vii. DOI: 10.1016/j.rdc.2014.05.003. PMID: 25034153. PMCID: PMC4198387.
Article6. Kalok A, Abdul Cader R, Indirayani I, Abdul Karim AK, Shah SA, Mohamed Ismail NA, et al. 2019; Sep. 25. Pregnancy outcomes in systemic lupus erythematosus (SLE) women. Horm Mol Biol Clin Investig. [Epub]. DOI:10.1515/hmbci-2019-0007. DOI: 10.1515/hmbci-2019-0007. PMID: 31553696.
Article7. Wincup C, McDonnell TCR, Rahman A. 2019; Menorrhagia: an underappreciated problem in pre-menopausal women with systemic lupus erythematosus. Lupus. 28:916–7. DOI: 10.1177/0961203319851868. PMID: 31132915. PMCID: PMC6566458.
Article8. Tsokos GC, Lo MS, Costa Reis P, Sullivan KE. 2016; New insights into the immunopathogenesis of systemic lupus erythematosus. Nat Rev Rheumatol. 12:716–30. DOI: 10.1038/nrrheum.2016.186. PMID: 27872476.
Article9. Leonard D, Eloranta ML, Hagberg N, Berggren O, Tandre K, Alm G, et al. 2016; Activated T cells enhance interferon-α production by plasmacytoid dendritic cells stimulated with RNA-containing immune complexes. Ann Rheum Dis. 75:1728–34. DOI: 10.1136/annrheumdis-2015-208055. PMID: 26493814.
Article10. Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A. 2002; Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 416:603–7. DOI: 10.1038/416603a. PMID: 11948342.
Article11. Klonowska-Szymczyk A, Wolska A, Robak T, Cebula-Obrzut B, Smolewski P, Robak E. 2014; Expression of toll-like receptors 3, 7, and 9 in peripheral blood mononuclear cells from patients with systemic lupus erythematosus. Mediators Inflamm. 2014:381418. DOI: 10.1155/2014/381418. PMID: 24692849. PMCID: PMC3955595.
Article12. Celhar T, Magalhães R, Fairhurst AM. 2012; TLR7 and TLR9 in SLE: when sensing self goes wrong. Immunol Res. 53:58–77. DOI: 10.1007/s12026-012-8270-1. PMID: 22434514.
Article13. Christensen SR, Kashgarian M, Alexopoulou L, Flavell RA, Akira S, Shlomchik MJ. 2005; Toll-like receptor 9 controls anti-DNA autoantibody production in murine lupus. J Exp Med. 202:321–31. DOI: 10.1084/jem.20050338. PMID: 16027240. PMCID: PMC2212997.
Article14. Chauhan SK, Singh VV, Rai R, Rai M, Rai G. 2013; Distinct autoantibody profiles in systemic lupus erythematosus patients are selectively associated with TLR7 and TLR9 upregulation. J Clin Immunol. 33:954–64. DOI: 10.1007/s10875-013-9887-0. PMID: 23564191.
Article15. Akira S, Uematsu S, Takeuchi O. 2006; Pathogen recognition and innate immunity. Cell. 124:783–801. DOI: 10.1016/j.cell.2006.02.015. PMID: 16497588.
Article16. Guiducci C, Gong M, Xu Z, Gill M, Chaussabel D, Meeker T, et al. 2010; TLR recognition of self nucleic acids hampers glucocorticoid activity in lupus. Nature. 465:937–41. DOI: 10.1038/nature09102. PMID: 20559388. PMCID: PMC2964153.
Article17. Kwon HK, Patra MC, Shin HJ, Gui X, Achek A, Panneerselvam S, et al. 2019; A cell-penetrating peptide blocks Toll-like receptor-mediated downstream signaling and ameliorates autoimmune and inflammatory diseases in mice. Exp Mol Med. 51:1–19. DOI: 10.1038/s12276-019-0244-0. PMID: 31028244. PMCID: PMC6486608.
Article18. Watson ML, Rao JK, Gilkeson GS, Ruiz P, Eicher EM, Pisetsky DS, et al. 1992; Genetic analysis of MRL-lpr mice: relationship of the Fas apoptosis gene to disease manifestations and renal disease-modifying loci. J Exp Med. 176:1645–56. DOI: 10.1084/jem.176.6.1645. PMID: 1460423. PMCID: PMC2119463.
Article19. Chen D, Hu W. 2018; Lupus podocytopathy: a distinct entity of lupus nephritis. J Nephrol. 31:629–34. DOI: 10.1007/s40620-017-0463-1. PMID: 29270846.
Article20. Celhar T, Lu HK, Benso L, Rakhilina L, Lee HY, Tripathi S, et al. 2019; TLR7 protein expression in mild and severe lupus-prone models is regulated in a leukocyte, genetic, and IRAK4 dependent manner. Front Immunol. 10:1546. DOI: 10.3389/fimmu.2019.01546. PMID: 31354711. PMCID: PMC6636428.
Article21. Suurmond J, Diamond B. 2015; Autoantibodies in systemic autoimmune diseases: specificity and pathogenicity. J Clin Invest. 125:2194–202. DOI: 10.1172/JCI78084. PMID: 25938780. PMCID: PMC4497746.
Article22. Moulton VR, Suarez-Fueyo A, Meidan E, Li H, Mizui M, Tsokos GC. 2017; Pathogenesis of human systemic lupus erythematosus: a cellular perspective. Trends Mol Med. 23:615–35. DOI: 10.1016/j.molmed.2017.05.006. PMID: 28623084. PMCID: PMC5650102.
Article23. Marshak-Rothstein A. 2006; Toll-like receptors in systemic autoimmune disease. Nat Rev Immunol. 6:823–35. DOI: 10.1038/nri1957. PMID: 17063184. PMCID: PMC7097510.
Article24. Nickerson KM, Christensen SR, Shupe J, Kashgarian M, Kim D, Elkon K, et al. 2010; TLR9 regulates TLR7- and MyD88-dependent autoantibody production and disease in a murine model of lupus. J Immunol. 184:1840–8. DOI: 10.4049/jimmunol.0902592. PMID: 20089701. PMCID: PMC4098568.
Article25. Celhar T, Fairhurst AM. 2014; Toll-like receptors in systemic lupus erythematosus: potential for personalized treatment. Front Pharmacol. 5:265. DOI: 10.3389/fphar.2014.00265. PMID: 25538618. PMCID: PMC4258990.
Article26. Christensen SR, Shupe J, Nickerson K, Kashgarian M, Flavell RA, Shlomchik MJ. 2006; Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity. 25:417–28. DOI: 10.1016/j.immuni.2006.07.013. PMID: 16973389.
Article27. Wu YW, Tang W, Zuo JP. 2015; Toll-like receptors: potential targets for lupus treatment. Acta Pharmacol Sin. 36:1395–407. DOI: 10.1038/aps.2015.91. PMID: 26592511. PMCID: PMC4816237.
Article28. Tilstra JS, Avery L, Menk AV, Gordon RA, Smita S, Kane LP, et al. 2018; Kidney-infiltrating T cells in murine lupus nephritis are metabolically and functionally exhausted. J Clin Invest. 128:4884–97. DOI: 10.1172/JCI120859. PMID: 30130253. PMCID: PMC6205402.
Article29. Liu J, Karypis G, Hippen KL, Vegoe AL, Ruiz P, Gilkeson GS, et al. 2006; Genomic view of systemic autoimmunity in MRLlpr mice. Genes Immun. 7:156–68. DOI: 10.1038/sj.gene.6364286. PMID: 16508641.
Article30. Kim YY, Park KT, Jang SY, Lee KH, Byun JY, Suh KH, et al. 2017; HM71224, a selective Bruton's tyrosine kinase inhibitor, attenuates the development of murine lupus. Arthritis Res Ther. 19:211. DOI: 10.1186/s13075-017-1402-1. PMID: 28950886. PMCID: PMC5615432.
Article31. Yung S, Chan TM. 2012; Autoantibodies and resident renal cells in the pathogenesis of lupus nephritis: getting to know the unknown. Clin Dev Immunol. 2012:139365. DOI: 10.1155/2012/139365. PMID: 22761629. PMCID: PMC3386553.
Article32. Rekvig OP. 2019; The dsDNA, anti-dsDNA antibody, and lupus nephritis: what we agree on, what must be done, and what the best strategy forward could be. Front Immunol. 10:1104. DOI: 10.3389/fimmu.2019.01104. PMID: 31156647. PMCID: PMC6529578.
Article33. Goilav B, Putterman C. 2015; The role of anti-DNA antibodies in the development of lupus nephritis: a complementary, or alternative, viewpoint? Semin Nephrol. 35:439–43. DOI: 10.1016/j.semnephrol.2015.08.005. PMID: 26573546. PMCID: PMC4662078.
Article34. Devarapu SK, Anders HJ. 2018; Toll-like receptors in lupus nephritis. J Biomed Sci. 25:35. DOI: 10.1186/s12929-018-0436-2. PMID: 29650017. PMCID: PMC5898010.
Article35. Conti F, Spinelli FR, Truglia S, Miranda F, Alessandri C, Ceccarelli F, et al. 2016; Kidney expression of Toll like receptors in lupus nephritis: quantification and clinicopathological correlations. Mediators Inflamm. 2016:7697592. DOI: 10.1155/2016/7697592. PMID: 27635115. PMCID: PMC5011205.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Lupus Enteritis That Developed during the Treatment of Systemic Lupus Erythematosus
- A Case of Transverse Myelitis as a First Manifestation of Systemic Lupus Erythematosus
- A Case Of Systemic Lupus Erythematosus Associated With Hyperthyroidism And Severe Retinopathy
- Multiple Dermatofibromas in a woman with Systemic Lupus Erythematosus
- A Case of Infectious Mononucleosis-like Syndrome Induced by Ceftriaxone and Isepamicin in a Patient with Systemic Lupus Erythematosus