Ann Hepatobiliary Pancreat Surg.
2021 May;25(2):206-214. 10.14701/ahbps.2021.25.2.206.
Adjuvant therapy using ex vivo-expanded allogenic natural killer cells in hepatectomy patients with hepatitis B virus related solitary hepatocellular carcinoma: MG4101 study
- Affiliations
-
- 1Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 2Cell Therapy Research Center, GC LabCell, Yongin, Korea
- 3Laboratory of Immunology and Infectious Diseases, Graduate School of Medical Science and Engineering, Korea Advanced Institute of Science and Technology, Daejeon, Korea
- KMID: 2516241
- DOI: http://doi.org/10.14701/ahbps.2021.25.2.206
Abstract
- Backgrounds/Aims
Fewer reports have been published regarding hepatectomy patients with solitary hepatocellular carcinoma (HCC) who received immunotherapeutic agents as adjuvant therapy. We evaluated the safety and efficacy of ex vivo-expanded allogenic natural killer (NK) cells in those patients with modified International Union Against Cancer (UICC) stage T3.
Methods
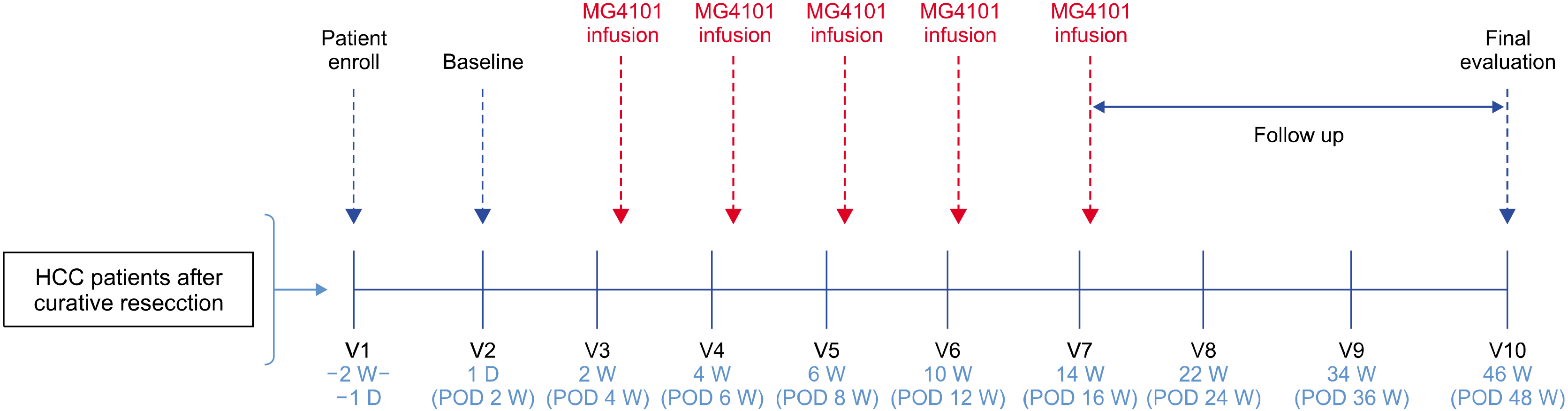
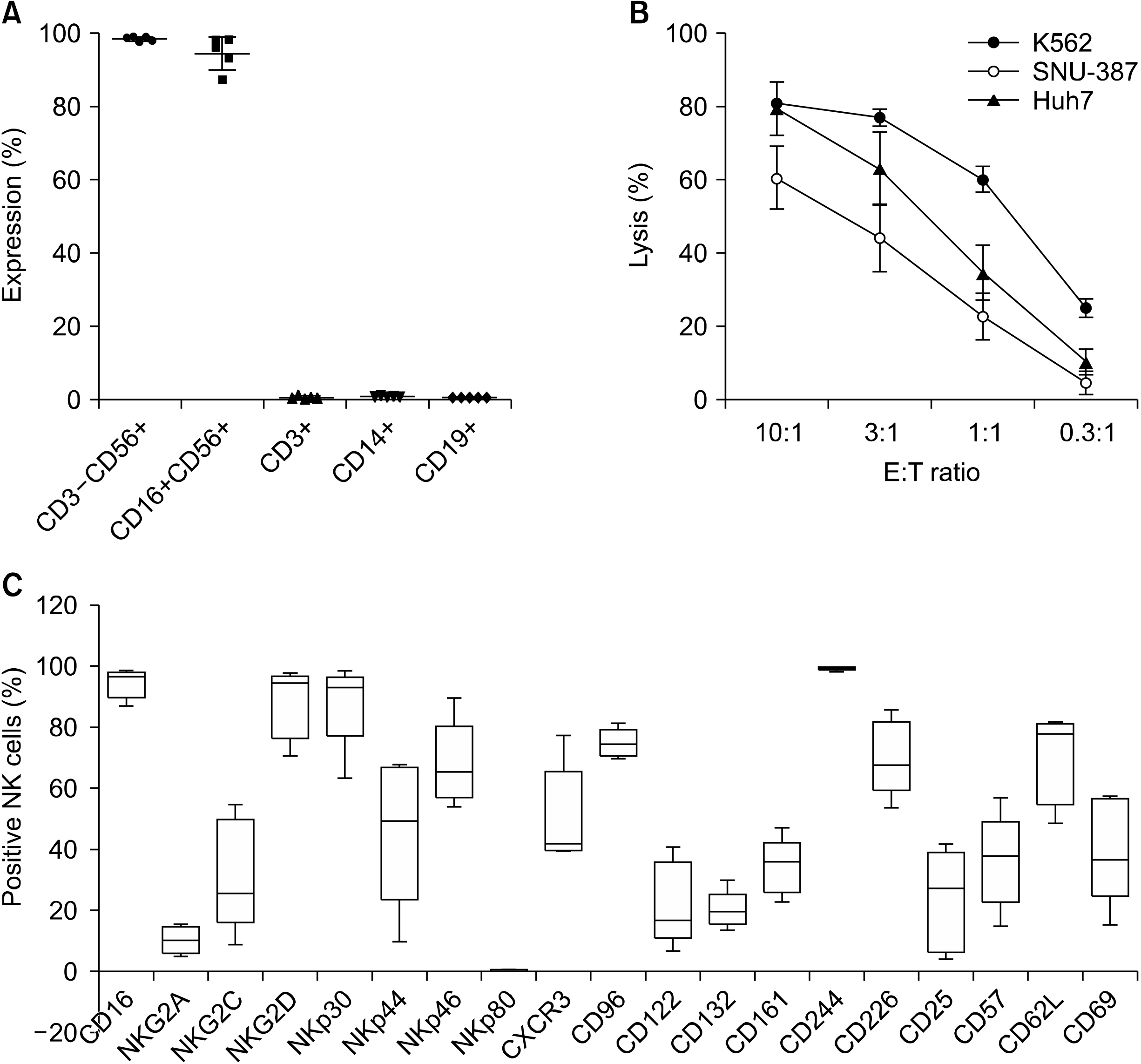
From August 2014 to October 2015, five patients who underwent hepatic resection received ex vivo-expanded allogenic NK cells. Patients received five rounds of NK cells (2-3×107 cells/kg) at postoperative 4, 6, 8, 12, and 16 weeks. This study is registered with ClinicalTrials.gov, number NCT02008929.
Results
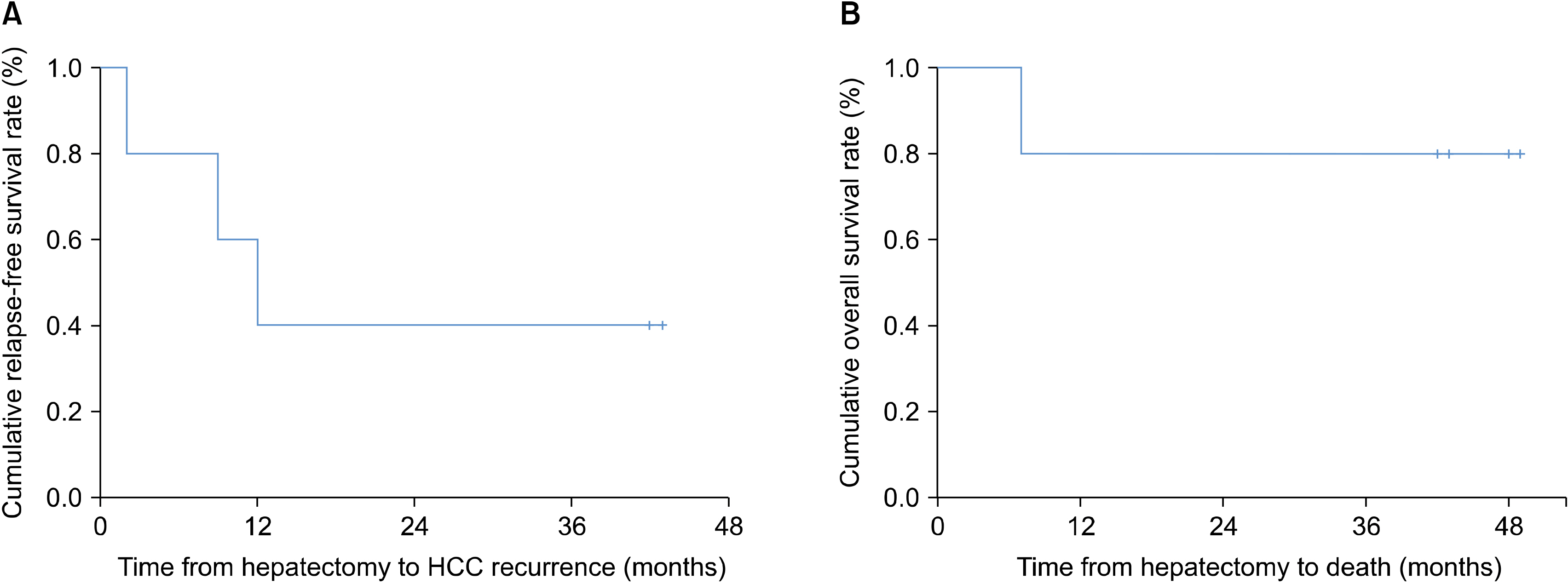
The median age of the five patients (three men and two women) was 44.8 years (range, 36-54 years). All had hepatitis B virus-related HCC, and the median tumor size was 2.2 cm (range, 2.1-8.2 cm). None of the patients had any adverse events. HCC recurrence developed in two patients at one year after hepatic resection, but four patients were alive at 3 years. The two recurrence-free patients showed a higher ratio of CD8+ T lymphocyte populations before and after administration of ex vivo-expanded allogenic NK cells compared with the three patients who experienced recurrence.
Conclusions
Immunotherapy using ex vivo-expanded allogenic NK cells in hepatectomy patients can be used safely. Further studies should be investigated for efficacy.
Figure
Reference
-
1. Yu SJ. 2016; A concise review of updated guidelines regarding the management of hepatocellular carcinoma around the world: 2010-2016. Clin Mol Hepatol. 22:7–17. DOI: 10.3350/cmh.2016.22.1.7. PMID: 27044761. PMCID: PMC4825164.
Article2. Kim JM, Kwon CH, Joh JW, Park JB, Lee JH, Kim SJ, et al. 2014; Outcomes after curative hepatectomy in patients with non-B non-C hepatocellular carcinoma and hepatitis B virus hepatocellular carcinoma from non-cirrhotic liver. J Surg Oncol. 110:976–981. DOI: 10.1002/jso.23772. PMID: 25171344.
Article3. Kim JM, Kwon CH, Joh JW, Park JB, Lee JH, Kim SJ, et al. 2014; Differences between hepatocellular carcinoma and hepatitis B virus infection in patients with and without cirrhosis. Ann Surg Oncol. 21:458–465. DOI: 10.1245/s10434-013-3302-1. PMID: 24132624.
Article4. Kim JM, Yi NJ, Kwon CHD, Lee KW, Suh KS, Joh JW. 2018; Early disseminated recurrence after liver resection in solitary hepatocellular carcinoma. Ann Surg Treat Res. 94:129–134. DOI: 10.4174/astr.2018.94.3.129. PMID: 29520346. PMCID: PMC5842084.
Article5. Jung SM, Kim JM, Choi GS, Kwon CHD, Yi NJ, Lee KW, et al. 2019; Characteristics of early recurrence after curative liver resection for solitary hepatocellular carcinoma. J Gastrointest Surg. 23:304–311. DOI: 10.1007/s11605-018-3927-2. PMID: 30215196.
Article6. Samuel M, Chow PK, Chan Shih-Yen E, Machin D, Soo KC. 2009; Neoadjuvant and adjuvant therapy for surgical resection of hepatocellular carcinoma. Cochrane Database Syst Rev. 2009:CD001199. DOI: 10.1002/14651858.CD001199.pub2. PMID: 19160192. PMCID: PMC7096780.
Article7. Bruix J, Takayama T, Mazzaferro V, Chau GY, Yang J, Kudo M, et al. 2015; Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double- blind, placebo-controlled trial. Lancet Oncol. 16:1344–1354. DOI: 10.1016/S1470-2045(15)00198-9.8. Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. 2018; AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 67:358–380. DOI: 10.1002/hep.29086. PMID: 28130846.
Article9. European Association for the Study of the Liver. 2018; Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 69:182–236.10. Korean Liver Cancer Study Group (KLCSG). National Cancer Center, Korea (NCC). 2015; 2014 KLCSG-NCC Korea Practice Guideline for the management of hepatocellular carcinoma. Gut Liver. 9:267–317. DOI: 10.5009/gnl14460. PMID: 25918260. PMCID: PMC4413964.11. Lee JH, Lee JH, Lim YS, Yeon JE, Song TJ, Yu SJ, et al. 2015; Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology. 148:1383–1391.e6. DOI: 10.1053/j.gastro.2015.02.055. PMID: 25747273.
Article12. Yu R, Yang B, Chi X, Cai L, Liu C, Yang L, et al. 2017; Efficacy of cytokine-induced killer cell infusion as an adjuvant immunotherapy for hepatocellular carcinoma: a systematic review and meta-analysis. Drug Des Devel Ther. 11:851–864. DOI: 10.2147/DDDT.S124399. PMID: 28360510. PMCID: PMC5364004.
Article13. Lee JH, Lee JH, Lim YS, Yeon JE, Song TJ, Yu SJ, et al. 2019; Sustained efficacy of adjuvant immunotherapy with cytokine-induced killer cells for hepatocellular carcinoma: an extended 5-year follow-up. Cancer Immunol Immunother. 68:23–32. DOI: 10.1007/s00262-018-2247-4. PMID: 30232520. PMCID: PMC6326973.
Article14. Moretta A, Pende D, Locatelli F, Moretta L. 2009; Activating and inhibitory killer immunoglobulin-like receptors (KIR) in haploidentical haemopoietic stem cell transplantation to cure high-risk leukaemias. Clin Exp Immunol. 157:325–331. DOI: 10.1111/j.1365-2249.2009.03983.x. PMID: 19664139. PMCID: PMC2745025.
Article15. Lim O, Jung MY, Hwang YK, Shin EC. 2015; Present and future of allogeneic natural killer cell therapy. Front Immunol. 6:286. DOI: 10.3389/fimmu.2015.00286. PMID: 26089823. PMCID: PMC4453480.
Article16. Yang Y, Lim O, Kim TM, Ahn YO, Choi H, Chung H, et al. 2016; Phase I study of random healthy donor-derived allogeneic natural killer cell therapy in patients with malignant lymphoma or advanced solid tumors. Cancer Immunol Res. 4:215–224. DOI: 10.1158/2326-6066.CIR-15-0118. PMID: 26787822.
Article17. Lim O, Lee Y, Chung H, Her JH, Kang SM, Jung MY, et al. 2013; GMP-compliant, large-scale expanded allogeneic natural killer cells have potent cytolytic activity against cancer cells in vitro and in vivo. PLoS One. 8:e53611. DOI: 10.1371/journal.pone.0053611. PMID: 23326467. PMCID: PMC3543306.
Article18. Grivennikov SI, Greten FR, Karin M. 2010; Immunity, inflammation, and cancer. Cell. 140:883–899. DOI: 10.1016/j.cell.2010.01.025. PMID: 20303878. PMCID: PMC2866629.
Article19. Hong YP, Li ZD, Prasoon P, Zhang Q. 2015; Immunotherapy for hepatocellular carcinoma: from basic research to clinical use. World J Hepatol. 7:980–992. DOI: 10.4254/wjh.v7.i7.980. PMID: 25954480. PMCID: PMC4419101.
Article20. Ungefroren H, Sebens S, Seidl D, Lehnert H, Hass R. 2011; Interaction of tumor cells with the microenvironment. Cell Commun Signal. 9:18. DOI: 10.1186/1478-811X-9-18. PMID: 21914164. PMCID: PMC3180438.
Article21. Zamarron BF, Chen W. 2011; Dual roles of immune cells and their factors in cancer development and progression. Int J Biol Sci. 7:651–658. DOI: 10.7150/ijbs.7.651. PMID: 21647333. PMCID: PMC3107473.
Article22. Korangy F, Höchst B, Manns MP, Greten TF. 2010; Immune responses in hepatocellular carcinoma. Dig Dis. 28:150–154. DOI: 10.1159/000282079. PMID: 20460904.
Article23. Harding JJ, El Dika I, Abou-Alfa GK. 2016; Immunotherapy in hepatocellular carcinoma: primed to make a difference? Cancer. 122:367–377. DOI: 10.1002/cncr.29769. PMID: 26540029.
Article24. Aerts M, Benteyn D, Van Vlierberghe H, Thielemans K, Reynaert H. 2016; Current status and perspectives of immune-based therapies for hepatocellular carcinoma. World J Gastroenterol. 22:253–261. DOI: 10.3748/wjg.v22.i1.253. PMID: 26755874. PMCID: PMC4698490.
Article25. Chew V, Chen J, Lee D, Loh E, Lee J, Lim KH, et al. 2012; Chemokine-driven lymphocyte infiltration: an early intratumoural event determining long-term survival in resectable hepatocellular carcinoma. Gut. 61:427–438. DOI: 10.1136/gutjnl-2011-300509. PMID: 21930732. PMCID: PMC3273680.
Article26. Lim KC, Chow PK, Allen JC, Chia GS, Lim M, Cheow PC, et al. 2011; Microvascular invasion is a better predictor of tumor recurrence and overall survival following surgical resection for hepatocellular carcinoma compared to the Milan criteria. Ann Surg. 254:108–113. DOI: 10.1097/SLA.0b013e31821ad884. PMID: 21527845.
Article27. Kim JM, Kwon CH, Joh JW, Park JB, Lee JH, Kim SJ, et al. 2013; PIVKA-II is a useful marker in patients with modified UICC T3 stage hepatocellular carcinoma. Hepatogastroenterology. 60:1456–1462.28. Imamura H, Matsuyama Y, Tanaka E, Ohkubo T, Hasegawa K, Miyagawa S, et al. 2003; Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 38:200–207. DOI: 10.1016/S0168-8278(02)00360-4.
Article29. Zarour HM. 2016; Reversing T-cell dysfunction and exhaustion in cancer. Clin Cancer Res. 22:1856–1864. DOI: 10.1158/1078-0432.CCR-15-1849. PMID: 27084739. PMCID: PMC4872712.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Natural Killer Cell Response to HCV Infection
- Two Cases of Early Recurred Hepatocellular Carcinoma after Surgical Resection Which Showed Different Outcomes
- The Correlation between NK Cell and Liver Function in Patients with Primary Hepatocellular Carcinoma
- A case of primary hepatocellular carcinoma following vertical transmission of hepatitis B virus in a child
- Optimization of Large-Scale Expansion and Cryopreservation of Human Natural Killer Cells for Anti-Tumor Therapy