J Korean Neurosurg Soc.
2021 May;64(3):386-405. 10.3340/jkns.2021.0023.
Disorders of Secondary Neurulation : Mainly Focused on Pathoembryogenesis
- Affiliations
-
- 1Neuro-oncology Clinic, Center for Rare Cancers, National Cancer Center, Goyang, Korea
- 2Division of Pediatric Neurosurgery, Seoul National University Children’s Hospital, Seoul, Korea
- 3Department of Anatomy, Seoul National University College of Medicine, Seoul, Korea
- KMID: 2515495
- DOI: http://doi.org/10.3340/jkns.2021.0023
Abstract
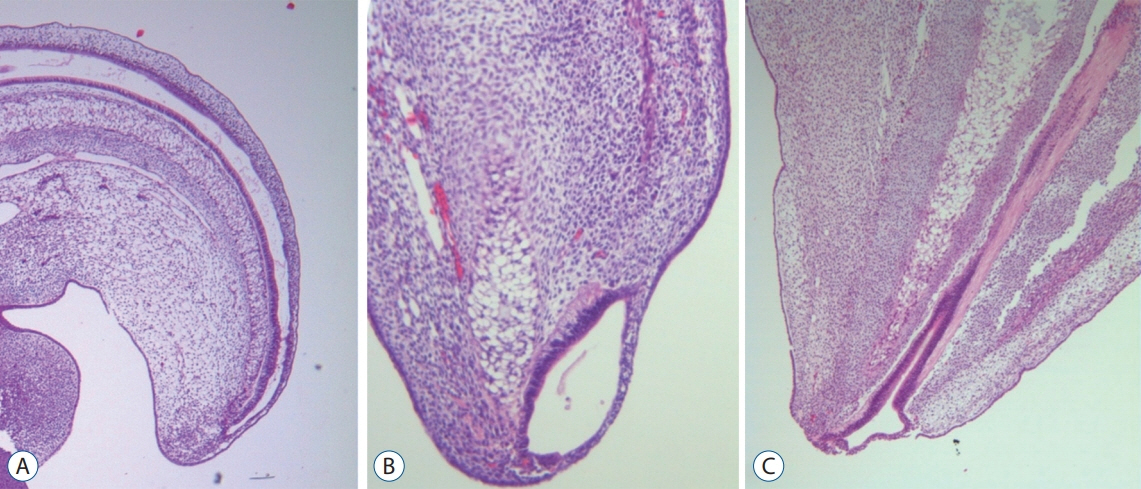
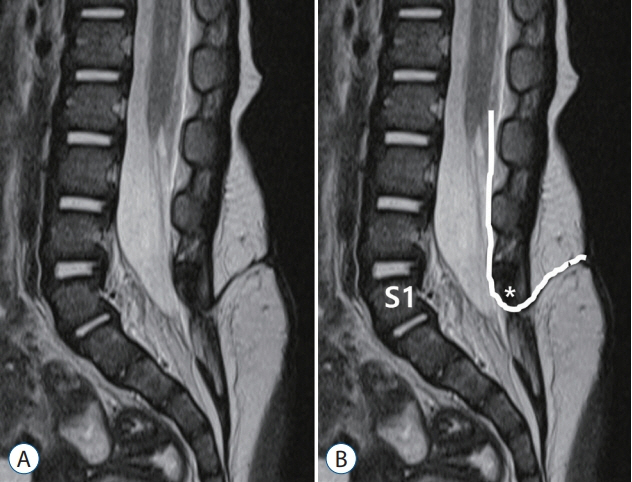
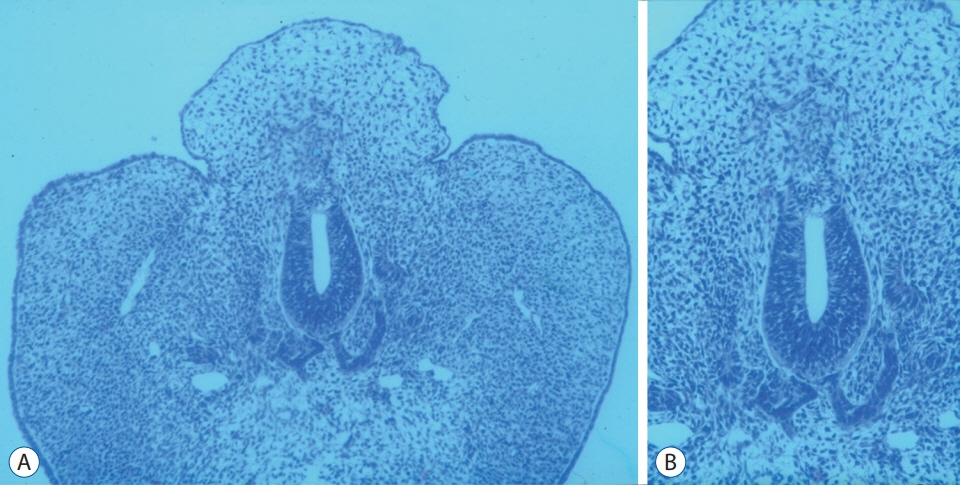
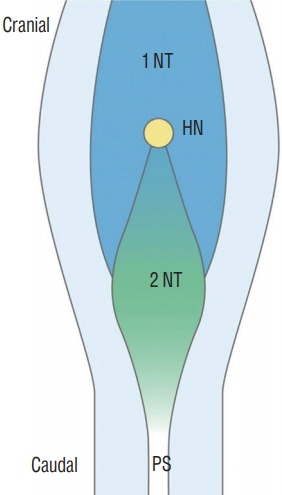
- Recent advancements in basic research on the process of secondary neurulation and increased clinical experience with caudal spinal anomalies with associated abnormalities in the surrounding and distal structures shed light on further understanding of the pathoembryogenesis of the lesions and led to the new classification of these dysraphic entities. We summarized the changing concepts of lesions developed from the disordered secondary neurulation shown during the last decade. In addition, we suggested our new pathoembryogenetic explanations for a few entities based on the literature and the data from our previous animal research. Disordered secondary neurulation at each phase of development may cause corresponding lesions, such as failed junction with the primary neural tube (junctional neural tube defect and segmental spinal dysgenesis), dysgenesis or duplication of the caudal cell mass associated with disturbed activity of caudal mesenchymal tissue (caudal agenesis and caudal duplication syndrome), failed ingression of the primitive streak to the caudal cell mass (myelomeningocele), focal limited dorsal neuro-cutaneous nondisjunction (limited dorsal myeloschisis and congenital dermal sinus), neuro-mesenchymal adhesion (lumbosacral lipomatous malformation), and regression failure spectrum of the medullary cord (thickened filum and filar cyst, low-lying conus, retained medullary cord, terminal myelocele and terminal myelocystocele). It seems that almost every anomalous entity of the primary neural tube may occur in the area of secondary neurulation. Furthermore, the close association with the activity of caudal mesenchymal tissue in secondary neurulation involves a wider range of surrounding structures than in primary neurulation. Although the majority of the data are from animals, not from humans and many theories are still conjectural, these changing concepts of normal and disordered secondary neurulation will provoke further advancements in our management strategies as well as in the pathoembryogenetic understanding of anomalous lesions in this area.
Figure
Reference
-
References
1. Acer T, Ötgün İ, Sağnak Akıllı M, Gürbüz EE, Güney LH, Hiçsönmez A. A newborn with caudal duplication and duplex imperforate anus. J Pediatr Surg. 48:E37–E43. 2013.
Article2. Al-Omari MH, Eloqayli HM, Qudseih HM, Al-Shinag MK. Isolated lipoma of filum terminale in adults: MRI findings and clinical correlation. J Med Imaging Radiat Oncol. 55:286–290. 2011.
Article3. Al Alayet YF, Samujh R, Lyngdoh TS, Mansoor K, Al Kasim F, Al-Mustafa AA. An extremely rare case of classic complete caudal duplication: dipygus. J Indian Assoc Pediatr Surg. 19:169–171. 2014.
Article4. Arai H, Sato K, Okuda O, Miyajima M, Hishii M, Nakanishi H, et al. Surgical experience of 120 patients with lumbosacral lipomas. Acta Neurochir (Wien). 143:857–864. 2001.
Article5. Balioğlu MB, Akman YE, Ucpunar H, Albayrak A, Kargın D, Atıcı Y, et al. Sacral agenesis: evaluation of accompanying pathologies in 38 cases, with analysis of long-term outcomes. Childs Nerv Syst. 32:1693–1702. 2016.
Article6. Bannykh SI, Bannykh GI, Mannino FL, Jones KL, Hansen L, Benirschke K, et al. Partial caudal duplication in a newborn associated with meningomyelocele and complex heart anomaly. Teratology. 63:94–99. 2001.
Article7. Bansal G, Ghosh D, George U, Bhatti W. Unusual coexistence of caudal duplication and caudal regression syndromes. J Pediatr Surg. 46:256–258. 2011.
Article8. Bertocchini F, Stern CD. Gata2 provides an early anterior bias and uncovers a global positioning system for polarity in the amniote embryo. Development. 139:4232–4238. 2012.
Article9. Brown E, Matthes JC, Bazan C 3rd, Jinkins JR. Prevalence of incidental intraspinal lipoma of the lumbosacral spine as determined by MRI. Spine (Phila Pa 1976). 19:833–836. 1994.
Article10. Chapman PH. Congenital intraspinal lipomas. Pediatr Neurosurg. 9:37–47. 1982.
Article11. Copp AJ, Stanier P, Greene ND. Neural tube defects: recent advances, unsolved questions, and controversies. Lancet Neurol. 12:799–810. 2013.
Article12. Criley BB. Analysis of embryonic sources and mechanims of development of posterior levels of chick neural tubes. J Morphol. 128:465–501. 1969.
Article13. Dady A, Havis E, Escriou V, Catala M, Duband JL. Junctional neurulation: a unique developmental program shaping a discrete region of the spinal cord highly susceptible to neural tube defects. J Neurosci. 34:13208–13221. 2014.
Article14. Denton JR. The association of congenital spinal anomalies with imperforate anus. Clin Orthop Relat Res. (162):91–98. 1982.
Article15. Dominguez R, Rott J, Castillo M, Pittaluga RR, Corriere JN Jr. Caudal duplication syndrome. Am J Dis Child. 147:1048–1052. 1993.
Article16. Economides KD, Zeltser L, Capecchi MR. Hoxb13 mutations cause overgrowth of caudal spinal cordand tail vertebrae. Dev Biol. 256:317–330. 2003.
Article17. Eibach S, Moes G, Hou YJ, Zovickian J, Pang D. Unjoined primary and secondary neural tubes: junctional neural tube defect, a new form of spinal dysraphism caused by disturbance of junctional neurulation. Childs Nerv Syst. 33:1633–1647. 2017.
Article18. Emery JL, Lendon RG. Lipomas of the cauda equina and other fatty tumours related to neurospinal dysraphism. Dev Med Child Neurol Suppl. 20:62–70. 1969.
Article19. Finger T, Schaumann A, Grillet F, Schulz M, Thomale UW. Retethering after transection of a tight filum terminale, postoperative MRI may help to identify patients at risk. Childs Nerv Syst. 36:1499–1506. 2020.
Article20. Gould GM, Pyle WL. Prenatal anomalies. Anomalies and curiosities of medicine. New York: The Julian Press, Inc;1896.21. Griffith CM, Sanders EJ. Effects of extracellular matrix components on the differentiation of chick embryo tail bud mesenchyme in culture. Differentiation. 47:61–68. 1991.
Article22. Griffith CM, Wiley MJ. Direct effects of retinoic acid on the development of the tail bud in chick embryos. Teratology. 39:261–275. 1989.
Article23. Griffith CM, Wiley MJ, Sanders EJ. The vertebrate tail bud: three germ layers from one tissue. Anat Embryol (Berl). 185:101–113. 1992.
Article24. Harris J, Blackwood B, Pillai S, Kanard R. Caudal duplication: management of a rare congenital condition. Am Surg. 82:E227–E229. 2016.
Article25. Hughes AF, Freeman RB. Comparative remarks on the development of the tail cord among higher vertebrates. J Embryol Exp Morphol. 32:355–363. 1974.
Article26. Irani N, Goud AR, Lowe LH. Isolated filar cyst on lumbar spine sonography in infants: a case-control study. Pediatr Radiol. 36:1283–1288. 2006.
Article27. Kim JW, Wang KC, Chong S, Kim SK, Lee JY. Limited dorsal myeloschisis: reconsideration of its embryological origin. Neurosurgery. 86:93–100. 2020.
Article28. Kim KH, Lee JY, Wang KC. Secondary neurulation defects-1 : retained medullary cord. J Korean Neurosurg Soc. 63:314–320. 2020.
Article29. Kim KH, Wang KC, Lee JY. Enlargement of extraspinal cysts in spinal dysraphism : a reason for early untethering. J Korean Neurosurg Soc. 63:342–345. 2020.
Article30. Kirby ML, Lawson A, Stadt HA, Kumiski DH, Wallis KT, McCraney E, et al. Hensen’s node gives rise to the ventral midline of the foregut: implications for organizing head and heart development. Dev Biol. 253:175–188. 2003.
Article31. Lee JY, Kim KH, Park K, Wang KC. Retethering : a neurosurgical viewpoint. J Korean Neurosurg Soc. 63:346–357. 2020.
Article32. Lee JY, Kim SP, Kim SW, Park SH, Choi JW, Phi JH, et al. Pathoembryogenesis of terminal myelocystocele: terminal balloon in secondary neurulation of the chick embryo. Childs Nerv Syst. 29:1683–1688. 2013.
Article33. Lee JY, Pang D, Wang KC. Caudal Agenesis and Associated Spinal Cord Malformations. In : Di Rocco C, Pang D, Rutka JT, editors. Textbook of Pediatric Neurosurgery. Cham: Springer International Publishing;2020. p. 2557–2575.34. Lee JY, Park SH, Chong S, Phi JH, Kim SK, Cho BK, et al. Congenital dermal sinus and limited dorsal myeloschisis: “spectrum disorders” of incomplete dysjuction between cutaneous and neural ectoderms. Neurosurgery. 84:428–434. 2019.
Article35. Lee JY, Phi JH, Kim SK, Cho BK, Wang KC. Urgent surgery is needed when cyst enlarges in terminal myelocystoceles. Childs Nerv Syst. 27:2149–2153. 2011.
Article36. Li YC, Shin SH, Cho BK, Lee MS, Lee YJ, Hong SK, et al. Pathogenesis of lumbosacral lipoma: a test of the “premature dysjunction” theory. Pediatr Neurosurg. 34:124–130. 2001.
Article37. McLone DG, Naidich TP. Spinal dysraphism: experimental and clinical. In : Holtzman RN, Stein BM, editors. The tethered spinal cord. New York: Thieme;1985. p. 14–28.38. Mills CL, Bellairs R. Mitosis and cell death in the tail of the chick embryo. Anat Embryol (Berl). 180:301–308. 1989.
Article39. Moore KL, Persaud TVN, Torchia MG. The Developing Human-EBook: Clinically Oriented Embryology. Elsevier Health Sciences;2018. p. 193–223.40. Morota N, Ihara S, Ogiwara H. New classification of spinal lipomas based on embryonic stage. J Neurosurg Pediatr. 19:428–439. 2017.
Article41. Müller F, O’Rahilly R. The development of the human brain, the closure of the caudal neuropore, and the beginning of secondary neurulation at stage 12. Anat Embryol (Berl). 176:413–430. 1987.
Article42. Müller F, O’rahilly R. The primitive streak, the caudal eminence and related structures in staged human embryos. Cells Tissues Organs. 177:2–20. 2004.
Article43. Muecke EC. The role of the cloacal membrane in exstrophy: the first successful experimental study. J Urol. 92:659–667. 1964.44. Nour S, Kumar D, Dickson JA. Anorectal malformations with sacral bony abnormalities. Arch Dis Child. 64:1618–1620. 1989.
Article45. Ostling LR, Bierbrauer KS, Kuntz C 4th. Outcome, reoperation, and complications in 99 consecutive children operated for tight or fatty filum. World Neurosurg. 77:187–191. 2012.
Article46. Pang D. Sacral agenesis and caudal spinal cord malformations. Neurosurgery. 32:755–778. discussion 778-779. 1993.
Article47. Pang D, Chong S, Wang K. Secondary neurulation defects-1: thickened filum terminale, retained medullary cord. In : Di Rocco C, Pang D, Rutka JT, editors. Textbook of pediatric neurosurgery. ed 1. Switzerland: Springer;2020. p. 1–18.48. Pang D, Dias MS, Ahab-Barmada M. Split cord malformation: part I: a unified theory of embryogenesis for double spinal cord malformations. Neurosurgery. 31:451–480. 1992.49. Pang D, Zovickian J, Lee JY, Moes GS, Wang KC. Terminal myelocystocele: surgical observations and theory of embryogenesis. Neurosurgery. 70:1383–1404. discussion 1404-1405. 2012.50. Pang D, Zovickian J, Moes GS. Retained medullary cord in humans: late arrest of secondary neurulation. Neurosurgery. 68:1500–1519. discussion 1519. 2011.
Article51. Pang D, Zovickian J, Oviedo A. Long-term outcome of total and neartotal resection of spinal cord lipomas and radical reconstruction of the neural placode: part I-surgical technique. Neurosurgery. 65:511–528. discussion 528-529. 2009.52. Pang D, Zovickian J, Oviedo A, Moes GS. Limited dorsal myeloschisis: a distinctive clinicopathological entity. Neurosurgery. 67:1555–1579. discussion 1579-1580. 2010.
Article53. Sadler TW, Feldkamp ML. The embryology of body wall closure: relevance to gastroschisis and other ventral body wall defects. Am J Med Genet C Semin Med Genet. 148C:180–185. 2008.
Article54. Sala F, Barone G, Tramontano V, Gallo P, Ghimenton C. Retained medullary cord confirmed by intraoperative neurophysiological mapping. Childs Nerv Syst. 30:1287–1291. 2014.
Article55. Schoenwolf GC. Tail (end) bud contributions to the posterior region of the chick embryo. J Exp Zool. 201:227–245. 1977.
Article56. Schumacher S. Über Bildungs-und Rückbildungsvorgänge am Schwanzende des Medullarrohres bei älteren Hühnerembryonen mit besonderer Berücksichtigung des Auftretens eines „sekundären hinteren Neuroporus“. Z Mikrosk Anat Forsch. 13:269–327. 1928.57. Scott RM, Wolpert SM, Bartoshesky LE, Zimbler S, Karlin L. Segmental spinal dysgenesis. Neurosurgery. 22:739–744. 1988.
Article58. Shimokita E, Takahashi Y. Secondary neurulation: fate-mapping and gene manipulation of the neural tube in tail bud. Dev Growth Differ. 53:401–410. 2011.
Article59. Streit A, Lee KJ, Woo I, Roberts C, Jessell TM, Stern CD. Chordin regulates primitive streak development and the stability of induced neural cells, but is not sufficient for neural induction in the chick embryo. Development. 125:507–519. 1998.
Article60. Sur A, Sardar SK, Paria A. Caudal duplication syndrome. J Clin Neonatol. 2:101–102. 2013.
Article61. Uehara M, Yashiro K, Takaoka K, Yamamoto M, Hamada H. Removal of maternal retinoic acid by embryonic CYP26 is required for correct Nodal expression during early embryonic patterning. Genes Dev. 23:1689–1698. 2009.
Article62. Wang KC. Spinal dysraphism in the last two decades : what I have seen during the era of dynamic advancement. J Korean Neurosurg Soc. 63:272–278. 2020.
Article63. Wang KC, Lee JS, Kim K, Im YJ, Park K, Kim KH, et al. Do junctional neural tube defect and segmental spinal dysgenesis have the same pathoembryological background? Childs Nerv Syst. 36:241–250. 2020.
Article64. Wei Y, Mikawa T. Fate diversity of primitive streak cells during heart field formation in ovo. Dev Dyn. 219:505–513. 2000.
Article65. Wong ST, Kan A, Pang D. Limited dorsal spinal nondisjunctional disorders: Limited dorsal myeloschisis, congenital spinal dermal sinus tract, and mixed lesions. Di Rocco C, Pang D, Rutka JT. Textbook of Pediatric Neurosurgery. ed 1. Switzerland: Springer;2020. p. 1–64.66. Yamada S, Won DJ, Pezeshkpour G, Yamada BS, Yamada SM, Siddiqi J, et al. Pathophysiology of tethered cord syndrome and similar complex disorders. Neurosurg Focus. 23:E6. 2007.
Article67. Yamada S, Won DJ, Yamada SM. Pathophysiology of tethered cord syndrome: correlation with symptomatology. Neurosurg Focus. 16:E6. 2004.
Article68. Yamada S, Zinke DE, Sanders D. Pathophysiology of “tethered cord syndrome”. J Neurosurg. 54:494–503. 1981.
Article69. Yang J, Kim KH, Lee JY, Wang KC. Caudal duplication syndrome: a literature review and reappraisal of its pthoembryogenesis. Childs Nerv Syst. 2021; [Epub ahead of print].70. Zhang T, Zhang HL, Wang da J, Tang XB, Jia HM, Bai YZ, et al. Normal development of hindgut and anorectum in human embryo. Int J Colorectal Dis. 26:109–116. 2011.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Terminal Myelocystocele : Pathoembryogenesis and Clinical Features
- Perspectives : The Role of Clinicians in Understanding Secondary Neurulation
- Secondary Neurulation Defects-1 : Retained Medullary Cord
- Junctional Neurulation : A Junction between Primary and Secondary Neural Tubes
- Overview of Secondary Neurulation